The mechanical properties of cathode materials are one of the key factors that directly affect the life of lithium-ion batteries. However, how to understand the exact mechanism of mechanical degradation of existing cathode materials remains a major challenge. To improve energy density, it is necessary to develop lithium-ion cathode materials with high reversible capacity and high operating voltage. In this regard, lithium transition metal oxides with layered structures and the general formula LiMO2 (where M = Ni, Co, and Mn) have been intensively studied because their electrochemical properties can be tuned by controlling the chemical composition of transition metals. By increasing the Ni content (≥60%) in the material, more Li+ can be reversibly present in the structure at higher operating voltages, which is controlled by the Ni2+/Ni4+ redox reaction. In practice, LiNixCoyMnzO2 (NCM) cathodes exhibit higher reversible capacity at high operating voltages compared to commercial LiCoO2. Recently, Professor Min-Sik Park of Kyung Hee University in South Korea and Ji-Sang Yu of the Advanced Battery Research Center of the Korea Institute of Electronics Technology cooperated to study the mechanical strength (such as particle hardness) of cathode materials (especially cathode materials with a layered structure). The relationship between the cycle performance was comparatively studied, and the team improved the particle hardness of the cathode material by magnesium doping, which could effectively suppress the formation of undesirable microcracks inside the particles during the cycle, thereby improving the cycle performance. To further elucidate the mechanical degradation mechanism of LIBs during repeated cycling and to improve the electrochemical performance and long-term durability of existing cathode materials, structural and electrochemical analyses were performed.
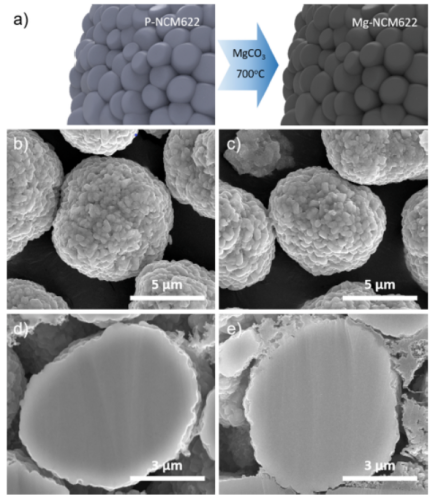
In order to study the relationship between particle hardness and the electrochemical performance of cathode materials, the authors synthesized P-NCM622 and Mg-NCM622 by conventional co-precipitation method. This method is to dope P-NCM622 with Mg through MgCO3 precursor, which can effectively improve mechanical properties of the particles, resulting in Mg-NCM622 with enhanced particle hardness. In a preliminary study, the Mg doping level was optimized by varying the amount of MgCO3 precursor (1, 2 and 3 wt%), as shown in Fig. 1a. Preliminary results show that Mg-NCM622 prepared with 1 wt% MgCO3 has the best performance. The FESEM images of P-NCM622 and Mg-NCM622 particles presented in Fig. 1b–c, respectively, demonstrate the successful synthesis of spherical secondary particles with an average size of about 8 μm, where each particle consists of nanoscale primary particles (300–500 nm) . Furthermore, the Mg-NCM622 particles have more planar surfaces compared to the P-NCM622 particles. This is attributed to the fact that the presence of magnesium promotes the crystal growth of primary particles. Furthermore, the cross-sectional FESEM images of the P-NCM622 and Mg-NCM622 particles presented in Fig. 1d–e, respectively, indicate that there is no significant difference between the particles.
Figure 1. Pristine and Mg-doped NCM622 particles: (a) schematic diagram of Mg doping process using MgCO3 precursor; (b) FESEM images of PNCM622 and (c) Mg-NCM622; (d) P-NCM622 and (e) ) Cross-sectional FESEM image of Mg-NCM622.
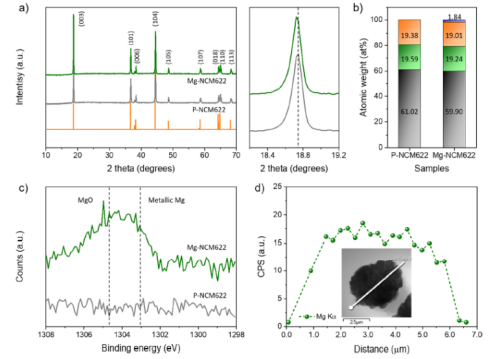
All reflections in the powder X-ray diffraction (XRD) patterns of P-NCM622 and Mg-NCM622 (Fig. 2a) well demonstrate that the structure is a hexagonal structure belonging to the R-3m space group. Mg doping leads to a shift of the (003) peak to low Bragg angles, with a small expansion of the c-lattice parameter. This dimensional change is mainly attributed to the partial Mg doping of the host structure. Furthermore, no impurity or undesirable formation of secondary phases such as MgO was detected in the map of Mg-NCM622. Furthermore, ICP-MS analysis confirmed the accurate content of Mg in the Mg-NCM622 particles to be 1.8 at.% (Fig. 2b). As expected, the Mg content in the host structure was increased by increasing the content of MgCO3 during the synthesis, which is consistent with the observed (003) XRD peak shift of Mg-NCM622.
Figure 2. Compositional characterization of P-NCM622 and Mg-NCM622 particles (a) Comparison of powder X-ray diffraction pattern and the (003) peak in magnification; (b) chemical composition determined by ICP-MS analysis; (c) XPS Mg 2s spectrum, and (d) the corresponding EDS line profile of Mg in Mg-NCM622.
The authors investigated the electrochemical behavior of P-NCM622 and Mg-NCM622 cathodes to elucidate the beneficial effects of magnesium doping. The galvanostatic voltage distributions of P-NCM622 and Mg-NCM622 cathodes in the range of 3.0–4.3 V vs. Li/Li+ under different galvanostatic conditions at 0.1, 0.2, 0.5, 1.0 and 2.0 C are shown in Fig. 3a–b . At a low current density of 0.1C (18 mA g-1), PNCM622 exhibits a voltage profile typical of a layered cathode material with a reversible capacity of 175 mAh g-1 and a first-time Coulombic efficiency of 99.0%. Mg-NCM622 has a similar electrochemical behavior with no obvious difference in its reversible capacity and initial Coulombic efficiency. Furthermore, both cathode materials show high reversible capacities of >150 mAh g-1 even at a high current density of 2.0 C (360 mA g-1). This indicates that under working conditions, Mg doping has no significant effect on the electrochemical performance of NCM622 in a single cycle. However, when cycling performance at a constant current density of 1.0 C (180 mA g-1) after 50 cycles (Fig. 3c), the P-NCM622 cathode showed a gradual capacity fading, while the Mg-NCM622 cathode maintained Stable cycle performance. After 50 cycles, the capacity retention of the Mg-NCM622 cathode was 96.2%, which was higher than that of PNCM622, which was 89.9%.
Summarize
This paper demonstrates the relationship between particle hardness and electrochemical performance of NCM622 cathode from a structural point of view. Experimental and theoretical studies have shown that Mg doping can enhance the mechanical strength (such as hardness) of NCM622 particles, thereby positively affecting the cycling performance of NCM622 cathodes. The doping of Mg effectively suppresses the formation of microcracks in the particles because Mg can enhance the structure of the particles during the repeated Li+ intercalation and deintercalation processes. Based on the correlation between particle hardness and electrochemical performance of P-NCM622 and Mg-NCM622 cathodes, the authors believe that particle hardness is one of the key factors determining the long-term cycling stability of NCM622 cathodes. Therefore, the results of this study will provide a practical guide for the development of high-performance cathode materials for lithium batteries. During the repeated intercalation and deintercalation of lithium ions, selective doping can improve the hardness of the particles and effectively suppress the microcracks inside the particles. Formation. Furthermore, this approach need not be limited to cathode materials with high nickel content; it is also applicable to most currently available cathode materials as well as cathode materials under development.