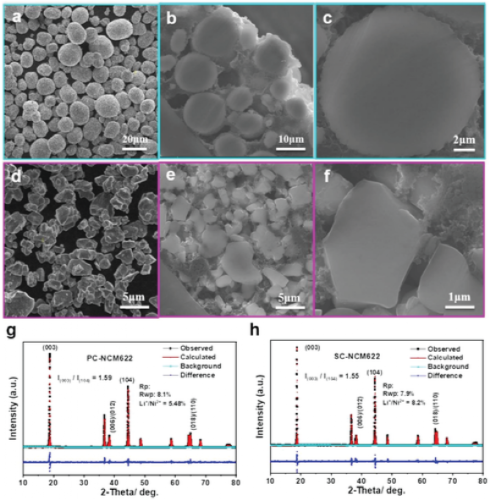
Li-ion batteries based on single-crystal LiNi1-x-yCoxMnyO2 (NCM, 1-x-y ≥ 0.6) cathode materials are gaining increasing attention due to their better structural stability compared to polycrystalline NCM-based batteries properties, resulting in a higher cycle life. However, in-depth understanding of the less obvious degradation mechanisms of single-crystal NCMs is still lacking.
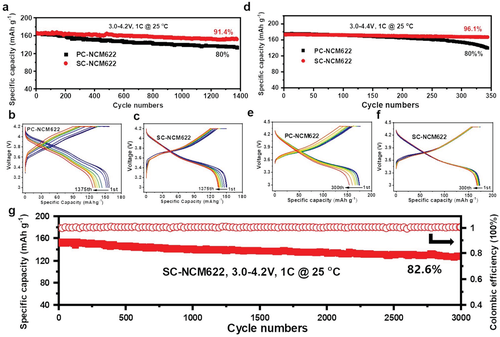
This work compares pouch cells with single- and polycrystalline LiNi0.60Co0.20Mn0.20O2 (NCM622) cathodes after 1375 discharge/charge cycles on graphite anodes. The thickness of the cationic disordered layer formed in the near-surface region of the cathode particles did not differ significantly between monocrystalline and polycrystalline particles, while cracks were evident in polycrystalline particles but almost absent in monocrystalline particles. Quantification of transition metal dissolution on the surface of the cycled graphite anode by time-of-flight mass spectrometry showed that the amount of dissolution of single-crystal NCM622 was greatly reduced. Likewise, quantified by electrochemical mass spectrometry, the carbon dioxide gas evolution of single-crystal NCM622 is also greatly reduced in the first two cycles. Benefiting from these advantages, the graphite/single crystal NMC622 pouch cell has a cathode areal capacity of 6 mAh cm-2 and a capacity retention rate of 83% after 3000 cycles to 4.2 V, emphasizing the single crystal NCM622 as a next-generation lithium-ion battery The potential of cathode materials.
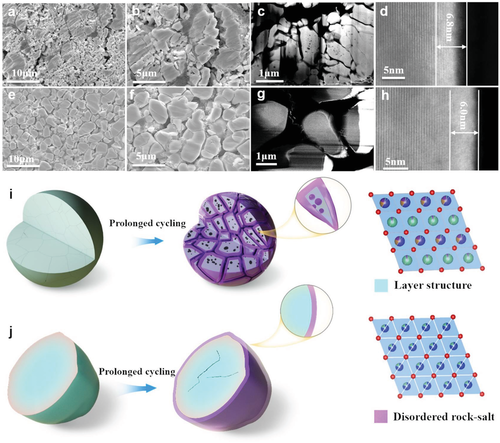
Figure 3-a,b) Ion-milled cross-sectional SEM images of SC-NCM622 and e,f) PC-NCM622 electrodes. HAADF-STEM images from the cycled c, d) PC-NCM622 and g, h) SC-NCM622 electrodes. i) Schematic illustration of the degradation of PC-NCM622 and j) SC-NCM622 particles after long-term cycling.
Figure 3a,b shows the ion-milled cross-sectional SEM images of the PC-NCM622 electrode at two different magnifications, showing severe crack formation, resulting in several fully collapsed secondary particles. Therefore, the formation of intergranular cracks is the main reason for the observed capacity fading and voltage polarization. The new surface is exposed to the electrolyte along the crack, exacerbating and accelerating the degradation. In addition to the cracks, holes can be seen in the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image in Fig. Interestingly, these cavities are located inside the primary particles, possibly created by the dissolution of transition metals. Particle crushing dominates the voltage polarization in PC-NCM622.
Figure 3i,j show the differences in the degradation mechanisms of PC-NCM622 and SC-NCM622 particles. After prolonged cycling, severe crack formation resulted in pulverization of PC-NCM622 particles, while only some microcracks appeared in SC-NCM622 particles. Furthermore, the cationic disordered layer (indicated in purple) is only formed on the surface of the SC-NCM622 particles, but penetrates into the interior of the PC-NCM622 particles along the intergranular cracks entering the electrolyte.
In conclusion
1. The research in this paper shows that the 2-4 μm SC-NCM622 particles are more robust to crack formation during cycling than the 10 μm PC-NCM622 particles, resulting in excellent long-term cycling stability of the pouch battery.
2. The thickness of the cationic disordered surface layer of SC-NCM622 and PC-NCM622 is comparable, but for PC-NCM622, the layer also forms along the intergranular cracks.
3. When PC-NCM622 was cycled, the transition metal dissolution detected on the surface of the SEI formed on graphite was much more pronounced than when SC-NCM622 was cycled.
4. In addition, the voltage polarization of PC-NCM622 increases more rapidly, which is mainly due to particle crushing caused by cracking of PC-NCM622 particles, which is also consistent with the CO evolution observed in the first two cycles.
5. The NCM622 material presented here does not use any dopants or protective layers, suggesting that the single-crystal approach is very effective in mitigating degradation at the cathode level.