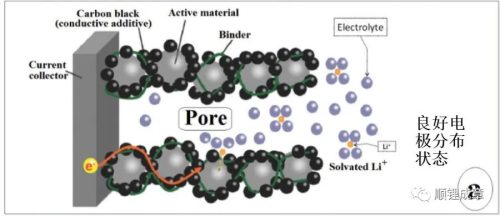
Lithium battery electrodes have a complex porous structure composed of AM (active material) and CA (conductive carbon black) particles, bound together with current collectors by binders.
The electrodes have the following requirements:
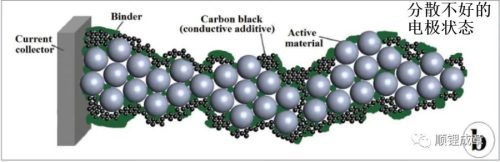
The AM is uniformly dispersed without agglomeration, and the CA particles should form an omnidirectional conductive network to maximize the electron channel between the AM and the current collector. In practical applications, CA is usually composed of various types of carbon black particles, and the electronic conductivity is comprehensively improved at multiple scales. The role of the binder is to ensure the mechanical stability of the electrode structure. The binder is usually a polyvinylidene fluoride (PVDF) based polymer series or SBR. Furthermore, the electrodes must be sufficiently porous to allow the electrolyte to contact all (or at least most) AM particles.
Lithium battery electrodes have a complex porous structure composed of AM (active material) and CA (conductive carbon black) particles, bound together with current collectors by binders.
The electrodes have the following requirements:
The AM is uniformly dispersed without agglomeration, and the CA particles should form an omnidirectional conductive network to maximize the electron channel between the AM and the current collector. In practical applications, CA is usually composed of various types of carbon black particles, and the electronic conductivity is comprehensively improved at multiple scales. The role of the binder is to ensure the mechanical stability of the electrode structure. The binder is usually a polyvinylidene fluoride (PVDF) based polymer series or SBR. Furthermore, the electrodes must be sufficiently porous to allow the electrolyte to contact all (or at least most) AM particles.
So for different material systems, is the mixing process universal?
In general, the shape and size of the AM have the same or greater effect on the conductivity and binder dispersion than the process parameters. As can be seen from the figure below, compared with the one-step mixing, the multi-step mixing may cause the formation of smaller AM soft agglomerates, and the conductivity can be increased by 1.35 times. 1.30 times). This suggests that the shape of the AM is affected by the sequential manner in which the slurry is blended. The slurry mixing sequence causes the modification of the electrode morphology and affects the electrode performance, which indicates that the slurry mixing process can be used as one of the effective ways to improve the electrode performance. The modification of electrode morphology is mainly the dispersion of AM and CB, and the distribution of AM/CB and binder. The solubility of the binder, the surface interaction of the binder with AM also plays an important role in the slurry. This shows that there will be differences for different pulp mixing methods.
Summarize
Slurry preparation seems to be very simple, but selection of specific electrode mixing process is required for specific AM and CA and binders, rather than selecting the general best slurry preparation method. The better mixing method for some systems may be ineffective for others. For these systems, the mixing process may destroy the composition of AM and CA, destroy the structure of the binder, and ultimately affect the electrode performance.
The prerequisite for preparing lithium electrode slurries is to obtain uniformity as well as proper distribution of the slurry components; only the proper combination of these slurry parameters can provide the correct method to improve the morphology of the electrode, thereby increasing the specific capacity and cycle life, and Minimize manufacturing costs. It is clear that the difficult task of preparing the slurry cannot be solved without extensive research into the application of different mixing and dispersion techniques in lithium electrode slurries.