Graphite is the most mature and widely used anode material at present, but its theoretical specific capacity is only 372 mAhg-1, and the room for improvement is limited. Currently, silicon-based anodes are considered to be the most promising next-generation anode materials, but they also suffer from problems such as volume expansion and poor cycling stability. Transition metal oxide materials have high energy density, good cycle performance and stable physical and chemical properties, and transition metal oxide materials have abundant reserves in the earth, low production cost and relatively simple preparation process, so they are also one of the popular materials in the field of anode materials. .
Among many transition metal oxides, tungsten oxide has attracted the attention of researchers due to its excellent semiconductor properties and potential application prospects. With the development of nanotechnology, tungsten oxide is also brought into the nano field. Compared with bulk tungsten oxide materials, nano-tungsten oxide is endowed with the common advantages of nano-materials: small size effect and quantum confinement effect. In addition, nano-tungsten oxide also has a variety of morphological structures. Due to its various advantages, nano-tungsten oxide has been widely studied in the fields of photocatalysis, electrochromism, gas sensing, and supercapacitors. Compared with carbon materials, nano-tungsten oxide has a higher theoretical specific capacity, so it has also attracted increasing attention in the field of lithium batteries.
1.Preparation of tungsten oxide
For the preparation of tungsten oxide, there are three main methods, namely liquid phase method, gas phase method and solid phase method.
liquid phase method
The liquid-phase method uses heating, stirring, etc. to make the solution chemically react in the liquid-phase environment. The product obtained by this method has high purity, uniform appearance, and the equipment used is simple, which can be used on a large scale. Therefore, this method is the most common and effective method for preparing WO3.
In addition, the liquid phase method also has the advantages of simple operation, simple equipment, wide operating temperature range, and the ability to prepare nanomaterials with various morphologies. Liquid-phase synthesis of WO3 can control the crystal structure and microstructure of the samples. There are various liquid-phase synthesis methods for tungsten oxide, such as the synthesis of WOx nanoparticles, nanowires, nanorods, nanosheets and nanotubes by sol-gel method, hydrothermal method and template method.
solid phase method
The solid-phase method is to uniformly mix the required raw materials according to a certain ratio, and then use high-temperature calcination or hot-pressing sintering to cause a solid-phase reaction between solid particles, which is a process method for preparing nano-powders. Sometimes it is further combined with grinding and pulverization technology to prepare the required ultra-fine nano powder.
The pulverization process of the solid phase method generally includes two types: one is to divide the bulk material into small particle size materials, including mechanical pulverization, etc., no material changes in the process. The second is to combine materials with small particle size, including spray pyrolysis, etc., there are material changes in the process.
The difference between the solid-phase method and the liquid-phase and gas-phase methods is that there is no liquid-solid or gas-solid transformation. Although the solid-phase method is simple, the obtained WO3 has low purity and is easy to agglomerate.
gas phase method
The vapor phase method can use physical vapor deposition (PVD), directly use tungsten wire, tungsten elemental powder, etc. as the tungsten source, and control the size and morphology of the deposited nano-WO3 particles by controlling the experimental parameters of vapor deposition. Most of the WO3 obtained by the gas phase method is WO3-x.
Compared with the solid-phase method, the gas-phase method has the advantages of small particle size, narrow distribution, good dispersibility, no impurities and low energy consumption. The WO3 product obtained by the gas phase method has high purity, but requires high experimental equipment, and is currently only suitable for laboratory, not suitable for industrial large-scale production.
2.Modification of Tungsten Oxide
When tungsten oxide is used as a negative electrode material for lithium batteries, although pure-phase WO3 exhibits better lithium storage performance than carbon materials, it also has shortcomings. In the first charge-discharge cycle of the material, the discharge specific capacity is very considerable, which basically exceeds the theoretical specific capacity of WO3 of 693mAhg-1, but in the subsequent cycles, most reports in the literature show that the capacity is not well maintained and decays. more obvious. Therefore, researchers have improved its electrochemical properties by coating methods, material composites, etc.
Zhang et al. used carbon nanotube film (CMF) as a flexible substrate to immobilize tungsten oxide (WO3) and carbon source (citric acid) on CMF by spraying method to form carbon-coated tungsten oxide/carbon nanotube film (WO3@C /CMF) composites. The materials were subsequently processed by freeze-drying and hydrothermal methods, and freeze-dried-carbon-coated tungsten oxide/carbon nanotube films (F-WO3@C/CMF) and hydrothermal-carbon-coated tungsten oxide films were obtained, respectively. /carbon nanotube films (H-WO3@C/CMF). It was found that WO3 in H-WO3@C/CMF had better dispersion.
Through the research on the mass ratio of tungsten source and carbon source, it is found that the electrochemical performance of H-WO3@C/CMF (1:1) obtained when the mass ratio of tungsten source and citric acid is 1:1 is better, and the first cycle discharge The specific capacity is 1180mAhg-1, and the discharge specific capacity is still 589mAhg-1 after 50 cycles. The results show that H-WO3@C/CMF is expected to improve its lithium storage performance as a negative electrode for Li-ion batteries.
Wang et al. successfully prepared a WO3/O2 composite film as a negative electrode material for lithium-ion batteries by using the micro-arc oxidation method, using titanium foil as the matrix and tungstate as the electrolyte. The structure of the composite membrane was characterized by scanning electron microscope and X-ray diffractometer. The composite membrane was mainly composed of WO3 and TiO2.
When the mass of sodium tungstate in the electrolyte is 70g, the electrochemical performance of the battery is the most stable, and the specific capacity is 605.684mAhg-1, and the specific capacity remains at 141.466mAhg-1 after 200 cycles. After coating with graphene, the initial specific capacity increased to 662.3mAhg-1, and the specific capacity remained at 614.1mAhg-1 after 200 cycles, the capacity retention rate was as high as 92.7%, and the electrochemical performance was better.
Yoon et al. prepared cauliflower-like WO3 by hydrothermal method and carbon-coated it. The cauliflower-like material is composed of small short rods with a length of about 50 nm and a diameter of 20 nm. When the current density of carbon-coated and uncoated materials is 50mAg-1, the discharge specific capacities after 50 cycles are 650mAhg-1 and 400mAhg-1, respectively, and with the continuous increase of the number of cycles, the properties of carbon-coated materials There is no attenuation but an improvement, while the material without carbon coating is more severe than the capacity attenuation.
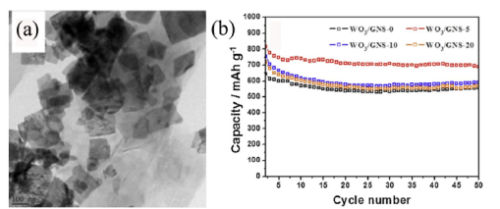
Kim et al. synthesized composites of WO3 nanoplates and graphene nanosheets by hydrothermal method, and measured the cycling performance of pure phase and composite 5wt%, 10wt% and 20wt% graphene under the condition of current density of 80mAg-1, respectively. curve, see Figure 1. The specific discharge capacity and cycle stability of composite graphene with different amounts are higher than those of pure WO3, and the best performance is obtained when the amount of composite graphene is 5wt%. The improvement of performance can be attributed to the addition of graphene to increase the electrical conductivity of the material, WO3 as an active material, graphene as an excellent electronic conductor, good contact, so that the performance of the electrode material has been improved.
Combining WO3 with other oxides also improves the electrochemical performance of the material. Gao et al. prepared WO3@SnO2 core-shell nanowire arrays by a two-step hydrothermal method, and the material maintained a capacity of 1000mAhg-1 after 200 cycles at a current density of 0.28C. The specific surface area of the composite material is doubled compared to the WO3 material before composite. The superior electrochemical performance of this composite nanostructure is attributed to the composite of SnO2, which reduces the overall internal resistance of the battery and improves the conductivity of the composite electrode. Stabilized WO3 nanostructures.
In short, tungsten oxide has the advantages of good chemical stability, environmental protection, low price and high capacity, and is a potential negative electrode material. However, the low electrical conductivity of tungsten oxide leads to its poor cycling stability, which limits its application as an anode material. With the rapid development of the new energy field, the research on tungsten oxide in the field of lithium battery is also deepening, and the future research results will be more and more abundant. I believe that tungsten oxide will also have a good development in lithium battery materials.