The application of Al2O3 in lithium-ion batteries has a long history. The most famous one is that the separator surface is coated with Al2O3 to improve the thermal stability of the separator, and the anode of Samsung SDI is coated with alumina to improve battery performance and safety. . However, there are more and more studies on the application of Al2O3 in lithium batteries recently. A little further, the research of the research group of Liu Zhaoping from the Ningbo Institute of Materials, Chinese Academy of Sciences shows that adding silane-Al2O3 to the conventional electrolyte can not only improve the electrochemical performance of the battery such as cycle and rate, but also effectively improve the safety of the battery. Then, the research of Dae Sik Kim of Korea and others showed that the graphite surface coated with amorphous Al2O3 can improve the fast charging performance of the battery. Recently, Jeff Dahn proposed a new insight that Al2O3 coated on the surface of NCM can react with LiPF6 to form LiPO2F2, which can improve battery performance.
Due to the trend of various factors, the capacity and energy density of power batteries continue to increase, and the safety of batteries is increasingly concerned. The safety issues of batteries are complex, but one of the root causes is the chemical system. Only by fundamentally improving the safety of materials can it be possible to improve the overall safety characteristics of batteries. Headquartered in Shenzhen, Betterray has a good reputation in the field of anode materials, and its R&D strength is second to none among domestic material factories. Recently, Dr. Xu Tao, who specializes in improving the safety of negative electrodes, proposed that Al2O3 can be coated on the surface of natural graphite by the sol-gel method, which can not only improve the cycle stability of the battery, but also improve the safety. The result is Synthesis of Alumina-Coated Natural Graphite for Highly Cycling Stability and Safety of Li-Ion Batteries was published in Chinese Journal of Chemistry.
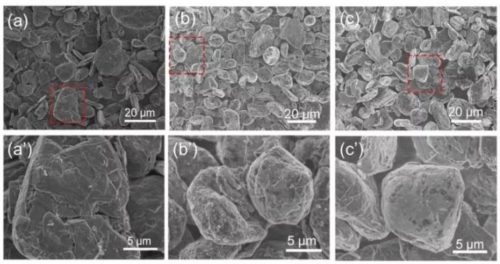
Figure 1. (a-c) SEM images of natural graphite (NG), 1 wt% Al2O3-coated natural graphite (denoted as AN-1), and 3 wt% Al2O3-coated natural graphite (denoted as AN-3), respectively ; (a’-c’) is the enlarged image of the boxed area in (a-c).
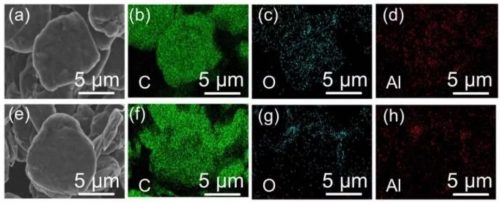
Figure 2. (a) and (e) are the SEM images of AN-1 and AN-3, respectively; (b-d) and (f-h) are the elemental distribution images of AN-1 and AN-3, respectively.
First, the authors coat Al2O3 on the surface of natural graphite (NG) by the sol-gel method. The preparation method of Sol-gel is very simple. First, add deionized water to Al(NO3)3·9H2O, then add natural graphite under stirring conditions, and finally dry to obtain natural graphite AN-1 (alumina-coated) coated with Al2O3. coating amount of 1 wt%) and AN-3 (alumina coating amount of 3 wt%). The SEM images of NG, AN-1 and AN-3 are shown in Figure 1, and the particle sizes of the three are about 11.55 μm, 11.58 μm and 11.67 μm, respectively. It can be seen from Figure 1 and Figure 2 that the Al2O3 coated on the surface of natural graphite by the sol-gel method is relatively uniform, but the natural graphite particles are not completely coated, but there are quite a few exposed areas.
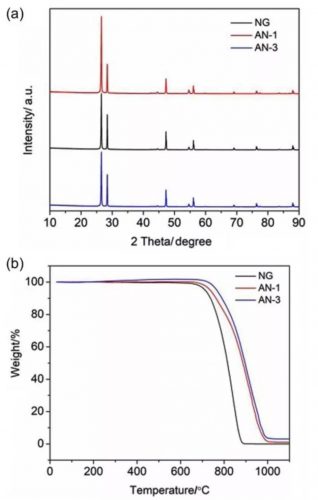
Figure 3. XRD patterns and TGA patterns of NG, AN-1 and AN-3.
The XRD patterns of AN-1 and AN-3 are almost the same as those of NG, and do not show the existence of Al2O3, which is mainly caused by too little coating of Al2O3. However, it can be clearly seen from the TGA curve that NG burns completely at 900 °C, while AN-1 and AN-3 still have mass, which is consistent with the amount of Al2O3 in them.
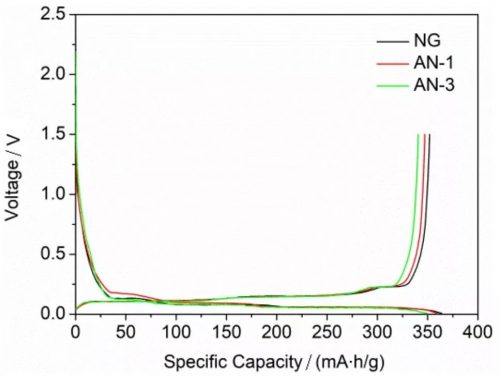
Figure 4. Charge-discharge curves of NG, AN-1 and AN-3 at 0.005-3 V and 0.1 C.
Figure 5. Charge-discharge curves of NG, AN-1 and AN-3 at 3.0-4.35 V and 1 C.
Subsequently, the authors conducted a comparative analysis of the electrochemical properties of NG, AN-1 and AN-3. First, in terms of discharge capacity, the discharge capacities of NG, AN-1, and AN-3 are 364.0, 359.8, and 350.4 mA/g, respectively, and the Coulombic efficiencies are 93%, 93.4%, and 93.5%, respectively. For the reduction of discharge capacity by Al2O3 coating, the author believes that the coating leads to the reduction of lithium ion diffusion channels; and the increase of Coulomb efficiency is caused by the reduction of side reactions by coating.
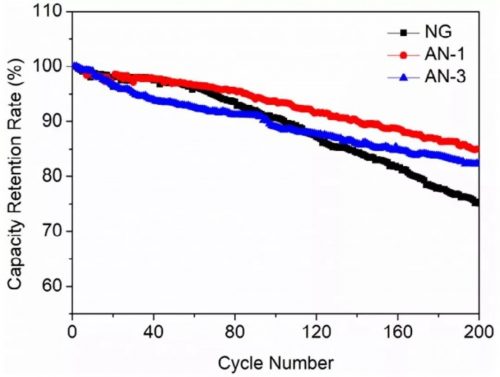
In this paper, the surface of natural graphite is coated with Al2O3 by the sol-gel method. The coating can not only inhibit side reactions, improve the cycle stability of the battery, but also improve the safety. The coating amount of Al2O3 is 1 wt% of natural graphite, and thermal runaway does not occur under the acupuncture experiment. Although the impact factor of this article is not high, it is still commendable to publish papers in enterprises, and the final battery safety improvement results are also very convincing.