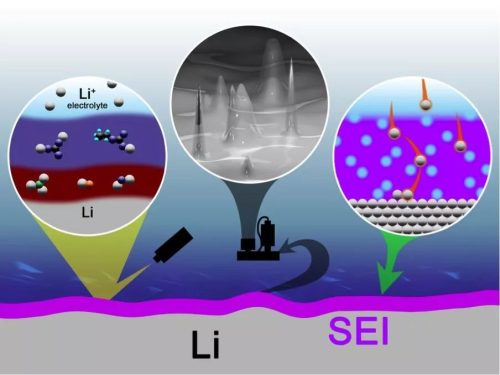
During the formation of lithium-ion batteries, the SEI film on the graphite surface of the negative electrode is mainly formed of inorganic lithium salts {Li2O, LiX (X=F, Cl, etc.)} in the early stage of formation, and the layer structure is relatively dense, and it is not suitable for the electrolyte. And the high temperature performance is more stable, while the outer layer of the SEI film is mainly composed of organic lithium salts (ROCO2Li, ROLi, etc.), the structure is loose, and the performance is not stable enough.
Lithium battery SEI film model diagram
Analysis of the influence of chemical charge cut-off voltage on battery performance
Influence of formation cut-off voltage of lithium cobalt oxide battery on battery performance
Lithium cobalt oxide (LiCoO2) is used as the positive active material and graphite is used as the negative active material to form a battery system, and the effect of the formation process on the battery performance is investigated by adjusting the formation charge cut-off voltage. The comparative experiment process is as follows:
1. Assemble the 683064 soft pack lithium-ion battery through the steps of mixing, coating, drying, assembling, liquid injection, standing, forming, and dividing.
2. The battery is divided into two groups, A/B. The cut-off voltage of group A is 3.8V, and the cut-off voltage of group B is 3.7V.
3. Comparative analysis of battery capacity, initial efficiency, energy density, internal resistance, rate, impedance, high temperature storage and cycle performance.
Basic performance comparison
The performance comparison results are shown in the following table. The capacity, specific capacity, initial efficiency, energy density, and battery gas production deformation rate of the battery formed by the low cut-off voltage are better than those of the high cut-off voltage, because the positive electrode specific capacity of the two schemes A/B is the same. , the difference of the whole battery mainly comes from the change of the negative electrode. The active lithium ion consumed by the B scheme is less than that of the A scheme, which makes the first efficiency and capacity higher, and the overall thickness is smaller.
Influence of formation depth of lithium iron phosphate battery on battery performance
The olivine-type LiFePO4 is used as the positive electrode material, graphite is used as the negative electrode material, and the ternary electrolyte is ethylene carbonate (EC), diethyl carbonate (DEC), and methyl ethyl carbonate (EMC) in a volume ratio of 1:1:1 A 1865140 square power battery with a rated capacity of 10Ah was assembled with LiPF6 as the supporting electrolyte of 1mol/L. The formation depth is controlled by adjusting the formation time, and the electrical properties of the battery are tracked and tested, and the influence of different formation depths on the electrical performance of the battery is analyzed.
By adjusting the formation charging time, the formation depths of the batteries of Groups I to IV are controlled to be 40%, 60%, 80% and 100% of the rated capacity, respectively.
The effect of high temperature cycle life of batteries
The cycle life curves of 1 C charge and discharge of each group of batteries in a constant temperature environment of 45 ℃ are shown in the figure below. The cycle life of the second to fourth groups of batteries is basically the same as the change rate of the number of charge and discharge, and the capacity of the fourth group of batteries remains after 100 cycles. The capacity of the battery of group I is relatively fast, especially after the 30th cycle, the capacity of the battery of group I has dropped significantly, and the capacity retention rate after 100 cycles is only 97.51%. It shows that when the formation depth reaches more than 60% of the rated capacity, continuing to deepen the formation depth has little effect on the high temperature cycle performance of the battery.
The effect of temperature on the formation results of lithium-ion batteries
In the process of formation, applying high temperature can reduce the viscosity of the electrolyte, accelerate the diffusion of ions, and ensure that electrons and ions are rapidly combined under high current; formation at high temperature can not only make the SEI film on the surface of the electrode react more fully, but also It can enhance the liquid absorption of the separator, which is beneficial to reduce the inflation of the battery.
However, high temperature formation will reduce the stability of the SEI film, resulting in poor battery cycle performance, because high temperature will aggravate the dissolution of the SEI film and the co-insertion of solvent molecules, while the SEI film tends to be stable at low temperatures. Studies have shown that the formation at low temperature is mainly based on solvent reduction, the reduction speed of lithium salts is slow, and the formation speed of SEI film is slow, so the deposition of solvent products is more orderly and dense, which is more conducive to prolonging the battery life.
In this paper, the flexible packaged lithium-ion battery of lithium cobalt oxide-graphite system is taken as the research object, and the effect of temperature on the formation effect in the high temperature pressure formation process is studied. The chemical composition process is divided into 4 cases, the temperatures are 50°C, 60°C, 80°C and temperature-variable chemical conversion. The variable-temperature chemical conversion refers to the two charging processes of chemical conversion with different temperatures. The first charging stage is 40°C, and the second charging stage The temperature during the charging phase was 70°C.
The performance of the first 350 cycles is similar, and the initial capacity can reach 3000 mA h. After 400 cycles, the capacity retention rate begins to differ. After 450 cycles, the capacities of schemes one, two, three and four remain The ratios are 90.57%, 90.63%, 93.44%, and 92.02%, respectively, indicating that the cycle performance of the 80°C high-temperature formation battery is better than that of the other solutions. It may be because the 80°C formation leads to greater battery activity and thicker SEI film. It is beneficial to the cycle stability of the battery.