A research project by nanoengineers at UC San Diego focused on recycling technologies for cathode batteries made from lithium iron phosphate. By reducing heavy metals such as nickel and cobalt, these types of batteries can help avoid the degradation of landscapes and water sources in mining fields, while avoiding exposure of workers to hazardous conditions while mining.
Most lithium batteries today use the rare and expensive metal cobalt as part of the cathode, but mining this material has a huge cost to the environment. Therefore, scientists have been working to find more environmentally friendly alternative materials, and lithium phosphate ion is one of them. According to reports, the latest research can also further improve its environmental performance, once it is used up, it can be restored to its original state using a small amount of energy.
Growing awareness of cobalt-related issues is driving a shift in the industry, with many looking to alternative battery designs, including big players like IBM and Tesla. This year, Tesla began selling the Model 3 with lithium iron phosphate batteries. These batteries are safer, last longer, and cost less to produce, but one of the downsides is that once they’re used up, they’re expensive to recycle.
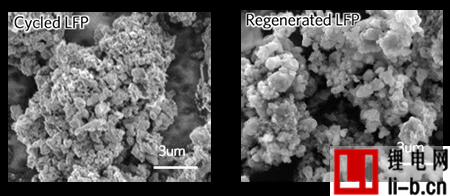
“It’s not cost-effective to recycle them,” said Zheng Chen, a professor of nanoengineering at UC San Diego. “It’s the same dilemma as plastics — the materials are cheap, but the way to recycle them is not.” Focuses on several mechanisms behind the performance degradation of LiFePO4 batteries. When they are cycled, this process drives structural changes, creating vacancies in the cathode as lithium ions are lost, while iron and lithium ions swap places in the crystal structure. This traps lithium ions and prevents them from circulating in the battery.
The research team took a commercially available lithium iron phosphate battery and consumed it to half its storage capacity. They then disassembled the battery and soaked the resulting powder in a solution containing lithium salts and citric acid, which was then washed, dried, and heated at temperatures of about 60 to 80°C. The powder was then made into new cathodes and tested in coin cells and pouch cells, where the team found that its performance returned to its original state.
The researchers note that this is because this recycling technique not only replenishes the battery’s lithium-ion reserves, but also allows the lithium and iron ions to return to their original positions in the cathode structure. This is thanks to the addition of citric acid, which provides electrons to the iron ions and reduces the positive charge that prevents the iron ions from moving to their original positions. The result of all this is that lithium ions can be released and cycled through the battery again. According to the study, the technology consumes 80 to 90 percent less energy and emits about 75 percent less greenhouse gases than current methods of recycling lithium-ion phosphate batteries. While this is a good start, the team says further research is needed to determine the overall environmental footprint of collecting and transporting large quantities of such batteries.
“The next challenge will be figuring out how to optimize these processes,” Zheng Chen said, “which will bring this recycling process closer to industry adoption.”