In order to meet the needs of high energy density of lithium-ion batteries, the proportion of active element nickel in NCM ternary materials has been increasing in recent years, and NCM9 series with nickel content of 90% and above have become the next-generation flagship products of many battery companies. Ternary cathode materials with high nickel content have higher initial discharge capacity, but they also bring a series of problems.
1. Insufficiency of high nickel NCM ternary materials
There is a trade-off between the specific capacity of NCM ternary cathode materials and capacity retention and thermal stability, with increasing Ni content, high Ni layered transition metal oxides provide higher specific capacity at the expense of capacity retention rate and thermal stability.
The main reason is that high nickel content will lead to an increase in the degree of cation mixing in the material, and high nickel ternary cathode materials are more likely to undergo irreversible phase transition from H2 phase to H3 phase during electrochemical cycling, and the degree of volume anisotropy changes is relatively high. Large, causing a series of problems such as material structure degradation, formation of microcracks, side reactions, and gas generation, resulting in a reduction in the cycle life of lithium-ion batteries.
Cation mix
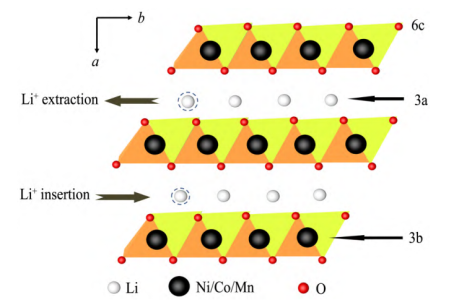
NCM ternary cathode materials are prone to cation mixing during the material synthesis stage and the electrochemical cycle process, that is, the transition metal ions at the 3a position and the lithium ions at the 3b position in the layered structure partially occupy the phenomenon.
The ionic radius of Ni2+ is 0.69 Å, and the ionic radius of Li+ is 0.76 Å. The two ionic radii are similar, and they are easy to occupy each other’s sites, resulting in the mixed arrangement of Li+/Ni2+.
During the sintering process of high-nickel ternary layered materials, Ni2+ in the material is difficult to be completely oxidized to Ni3+, and part of Ni2+ in the material may migrate from the transition metal layer in the layered structure to the Li+ layer to form Li+/Ni2+ mixed arrangement. During the electrochemical cycle, Li+ is released from the interlayer of the material, resulting in a large number of vacancies. Part of the Ni2+ in the 3a position will spontaneously migrate to the Li layer and occupy the Li+ position. Excessive Ni2+ occupying the Li layer will aggravate the layered structure to the spinel. The transformation of the rock structure or even the rock-salt phase structure results in serious capacity attenuation.
Crystal structure of layered NCM cathode material
surface side reactions
The NCM ternary cathode material will inevitably have side reactions in contact with the components in the air and the electrolyte during the synthesis and electrochemical cycling process, and the generated by-products will seriously affect the cycle stability and safety performance of the battery. In the preparation of high-Ni-N ternary layered materials, in order to obtain an ordered layered structure material and to compensate for the volatilization of the lithium source under high temperature conditions, the lithium source is usually excess relative to the transition metal during the mixed lithium sintering process, which will This results in the presence of residual lithium on the surface of the positive electrode material.
Therefore, if the high-nickel ternary cathode material is exposed to the air, the residual lithium on the surface of the material will react with CO2 and H2O in the air to form by-products such as LiOH, Li2CO3, etc., which hinder the migration of Li+, and will also interact with the electrolyte and polymer. The side reaction of the binder and the like and the generation of gas, etc., lead to the deterioration of the battery performance.
2. Al ion doping modification of high nickel NCM ternary material
Doping other elements in the crystal structure is a common method for material modification. Doping with trace elements can stabilize the structure of high-nickel NCM materials and improve the reversibility of electrode reactions.
Among the doping elements, Al element is the most widely studied. In a sense, NCM doped with Al element can be regarded as the marriage of NCM ternary and NCA ternary. Does it look like the quaternary lithium battery NCMA promoted by LG New Energy in South Korea?
Un-Hyuck Kim et al. of Hanyang University in South Korea developed a NCMA89 material (Li[Ni0.89Co0.05Mn0.05Al0.01]O2), and compared it with NCM90 and NCA89 to verify the Al element doping Effects on high nickel NCM.
Advantage 1: reduce costs
On the basis of ternary battery, NCMA replaces part of Co element with Al element, which can reduce the use of rare and toxic Co element and reduce the cost.
Advantage 2: Improve battery stability
The radius of Al3+ is similar to that of Co3+, and it is electrochemically inert. Even at high voltage, it can form a stable bond with the surrounding O2-, which can stabilize the structure and improve the cycle stability of the battery. The strength is stronger than the Ni(Co,Mn)-O bond, which is more inclined to improve thermal stability.
To further improve the electrochemical performance of NCM materials, it is necessary to solve the problems of Li/Ni mixing, formation of rock-salt phase and oxygen loss, surface residual lithium, interfacial side reactions and particle cracks. At present, the better solutions include element doping, surface coating and single crystal structure design.
Doping can stabilize the internal structure of the material by adjusting the chemical composition, thereby inhibiting Li/Ni mixing, formation of rock-salt phase and oxygen loss, but there are also some disadvantages, such as introducing impurity phase, reducing capacity, etc. Therefore, a reasonable choice of doping Impurity elements and doping levels are particularly important.
As one of the most studied doping elements, Al element has attracted the attention of many domestic and foreign manufacturers. In terms of NCMA battery industrialization, LG New Energy and Honeycomb Energy have become the leaders. In addition, Posco Chemical, Huayou Cobalt, Rongbai Technology, GEM, and Zhongwei are also in R&D and testing. Driven by leading companies, NCMA batteries are expected to increase rapidly in the next few years.