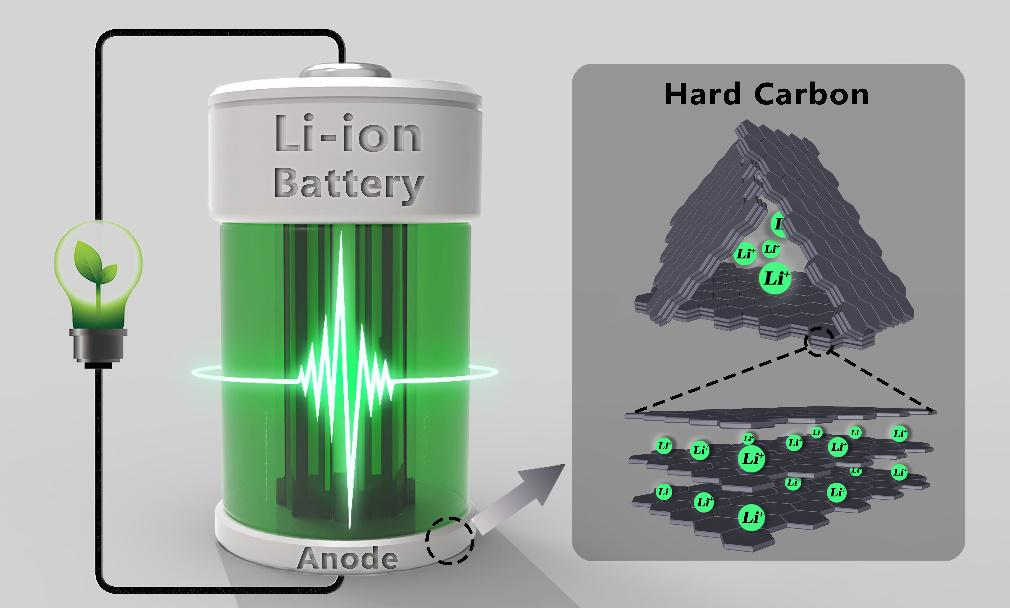
At present, the negative electrode of commercial lithium-ion batteries is mainly made of graphite materials. According to the lithium storage mechanism of LiC6 between graphite layers, its theoretical specific capacity is only 372 mAh/g, and the room for improvement is very limited, and lithium diffusion between graphite layers also restricts its rate performance. It can be seen that with the increasing demand for battery energy and power performance in downstream applications, pure graphite anode materials have become stretched. As a new type of negative electrode material, hard carbon has a lithium potential similar to graphite and a higher specific capacity. More importantly, hard carbon is composed of graphite-like crystallite structure and open horn-like crystallite, this unique crystallite structure can not only provide more lithium storage sites, but also facilitate lithium ion in the graphite layer. Between de-embedding. Therefore, as a new generation of lithium-ion battery anode materials, hard carbon has a very broad development prospect.
Hard carbon does not have a uniform structural model like graphite. Affected by different precursors and preparation conditions, the actual structure of hard carbon is very complex, and it is difficult to construct a general model. In 1951, Franklin believed that hard carbon is composed of some randomly arranged, localized graphitized structures, and is connected by amorphous carbon. Later, Ben proposed in 1975 that hard carbon is composed of some interlaced, curved, graphitized ribbon-like winding structures, but this model cannot explain why hard carbon cannot be further graphitized as the temperature increases. In 1997, Harris proposed that hard carbon has an isotropic three-dimensional structure similar to that of foam, and the formed microporous wall is composed of curved carbon layers with a fullerene-like structure, including five-membered rings and six-membered rings. The five-membered ring makes the carbon layer curved and irregular, and further high-temperature treatment cannot be graphitized. In recent years, although new progress has been made in the study of hard carbon structure models, the understanding of its structure and properties still needs to be deepened, and many models still need more reliable evidence support.
For hard carbon, the electrochemical intercalation of Li+ starts at about 0.8 Vvs Li/Li+, and the entire voltage curve has no obvious plateau, showing a gradual downward trend. Unlike graphite, there is no ordering phenomenon in the process of intercalation of Li+ into hard carbon, and the different electrochemical behaviors can be explained by their structural differences. With the advancement of in-situ characterization and computer simulation technology, people’s understanding of the storage mechanism of Li+ in hard carbon has been deepening. According to the existing Li+ ion storage mechanism, it can be basically divided into the following three categories: 1) Nanopores for Li+ 2) Adsorption of Li+ on defect sites, 3) Li+ intercalation into graphite layer.
Hard carbons used as anodes for lithium-ion batteries are mainly prepared using resin-based, pitch-based, and biomass-based precursors. The preparation methods and optimization strategies of hard carbon derived from the above three precursors are summarized in this paper.
Hard carbon is used as a key electrode material in lithium-ion batteries to achieve rapid intercalation and deintercalation of lithium ions during charge and discharge, and it has shown broad prospects in high-energy and high-power energy storage applications. In recent years, the research on hard carbon anode materials has made significant progress, but many challenges/bottlenecks still exist: 1) The lithium storage capacity of most hard carbon materials is still low; 2) The rate performance and cycle performance need to be improved; The first-week Coulombic efficiency of carbon anodes is usually low; 4) The lithium storage mechanism is not fully understood.
In order to meet the needs of practical battery applications, this paper proposes the following prospects for the future development of hard carbon anodes: 1) optimize the precursor; 2) optimize the micro/nano structure; 3) adopt advanced pre-lithiation technology; 4) develop 0 5) Develop low-temperature fast charging devices; 6) Focus on cost control, quality management and standard formulation, and promote industrial production.