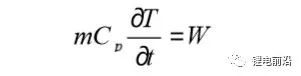Due to the high energy density of lithium-ion batteries, and the low thermal stability of positive and negative materials, the electrolyte is a flammable organic system. Therefore, lithium-ion batteries may have serious safety problems under certain conditions, such as high temperature or even Fire and explosion. There are various reasons for the safety problems of lithium-ion batteries, such as mechanical damage, environmental damage, electrical damage, and self-instability. Mechanical damage mainly refers to the deformation and rupture of the lithium-ion battery when it is subjected to external mechanical stress, resulting in battery short circuit, liquid leakage and burning; environmental damage refers to the safety problems caused by the lithium-ion battery working in an unsuitable environment. For example, lithium-ion batteries generate heat under high temperature conditions, and accidents such as fire and explosion occur when the heat accumulates to a certain level; the most common form of electrical damage is battery overcharging; in addition, lithium-ion batteries are caused by long-term use, such as lithium Changes in materials and results such as crystal growth may lead to internal short circuits in the battery and cause safety problems. However, no matter what the cause of the safety problem of lithium-ion batteries is, the final safety accidents of lithium-ion batteries are accompanied by internal and external short circuits, and the temperature rises sharply or even fires and explodes, that is, the thermal runaway of lithium-ion batteries.
With the large-scale application of lithium-ion batteries in the field of electric vehicles and energy storage, the battery modules used in electric vehicles are usually very large. For example, the battery of Nissan Leaf uses lithium manganate (LiMn2O4) as the positive electrode material. The battery module contains 192 A single battery of 34Ah, the entire battery module including the control circuit weighs 300kg. The Tesla Roadster’s battery module consists of 6,831 18650 cells. If the heat cannot be discharged to the outside of the battery module in a timely and sufficient manner due to design reasons or a cooling system failure, one or more single cells inside the module will accumulate heat. If the temperature of the battery eventually reaches the thermal runaway temperature, it may cause the battery to leak or burn, or even cause the battery to burst. The large-scale runaway phenomenon of the entire battery system caused by the thermal runaway of lithium-ion batteries is the thermal runaway extension of lithium-ion batteries. For large-capacity, high-power large-scale lithium-ion battery modules, safety issues are even more prominent. Since thermal runaway expansion occurs on large-scale lithium-ion battery modules, it is very difficult to put out a fire after a fire, which often causes casualties and large economic losses, and the impact is very large.
The research work on the thermal runaway expansion characteristics of lithium-ion batteries is very important. The reasons for thermal runaway expansion mainly include the stability of the battery itself, the thermal conductivity between the batteries, and the heat exchange conditions between the system and the environment. This paper focuses on the above content.
Specific heat capacity test
In order to calculate the electrochemical heat production and heat production rate of the lithium-ion battery, it is necessary to measure the specific heat capacity Cp of the battery. At the same time, in order to provide the basic parameters of thermal characteristics for the thermal model, the important thermodynamic parameter of the specific heat capacity is also required. The specific heat capacity test adopts the test method of constant power heating. Using the adiabatic environment provided by the accelerated adiabatic calorimeter (ARC), the heating plate provides constant power to the battery and heats it to 60 ℃ (if the temperature is higher, the battery will be damaged easily), you can get The battery temperature changes with time data, and then Cp is calculated by formula (1).
m is the sample mass; Cp is the specific heat capacity; W is the heating power. Test the specific heat capacity of ternary lithium-ion battery and lithium iron phosphate lithium-ion battery, the values are: 1245 and 1.239 J/(kg·K), the difference between the two is small.
Thermal runaway extension test
The test uses 5 cells to form a battery module. In order to avoid uncertain factors caused by the electrical connection between cells, the cells are not connected. The group form of the test battery is shown in Figure 1. In order to ensure that the parameters of the combination tightening state are consistent, the same 3N·m torque wrench is used during assembly.
Thermal runaway expansion of ternary lithium-ion battery modules
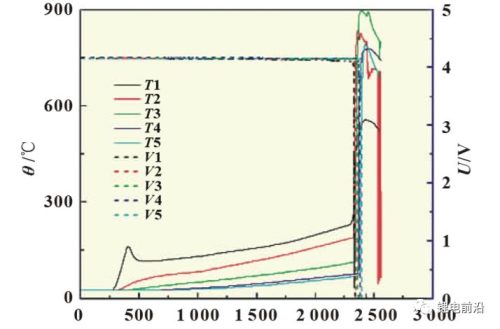
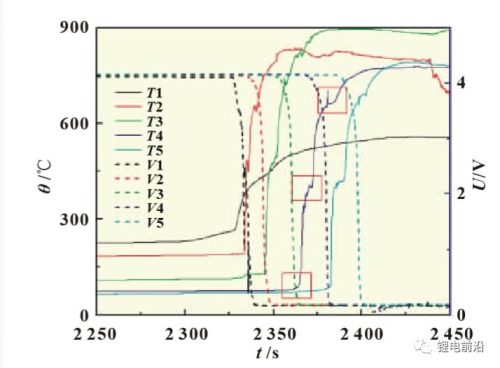
Figure 2 is the curve of the thermal runaway extension test of the ternary lithium-ion battery module, and Figure 3 is a partial enlargement of the curve. As can be seen from the figure, starting from 250s, the temperature measuring point T1 between the heating plate and the battery rises rapidly and exceeds 150°C, and then the temperature drops slightly, and the heating continues. The temperature gradually increased, reaching 270°C. At this time, the thermal runaway of the 1# battery occurs, the voltage drops to 0V, and the temperature measurement point T2 between the 1# and 2# batteries climbs rapidly, exceeding 800 °C. Before the thermal runaway occurs in the 1# battery, the different temperature measurement points T2, T3, T4 and T5 all have a certain degree of climb, showing a gradient distribution, which is related to the heat transfer between the batteries. Before the thermal runaway of the 1# battery, when the temperature of T1 was about 230 ℃, there was a rapid temperature rise in T1, and the voltage of the 1# battery fluctuated slightly, indicating that a slight short circuit occurred in the battery at this time. Subsequently, when the temperature of T1 was 270 °C and the temperature of T2 was 190 °C, the thermal runaway of the 1# battery occurred. Then 2#, 3#, 4#, and 5# batteries suffered thermal runaway in turn. Looking carefully at Figure 3, we can see that there is more than one sudden rise in temperature in the temperature curve. Taking the T4 curve in the figure as an example, the first temperature sudden rise corresponds to the occurrence of thermal runaway of the 3# battery. At this time, the voltage of the 3# battery drops to 0V, and a complete thermal runaway occurs; the second temperature sudden rise occurs. Corresponding to the thermal runaway of the 4# battery, at this time, the voltage of the 4# battery began to drop, and thermal runaway began to occur; the third temperature surge corresponds to the beginning of the thermal runaway of the 5# battery, and the voltage of the 5# battery began to drop. It can be seen from the temperature change of the battery that when the thermal runaway expansion occurs, due to the continuous thermal runaway of new batteries and the release of energy, the heat of the battery module accumulates in a short period of time, the temperature continues to rise, and the maximum temperature reaches almost 900 °C. Figure 4 shows photos of the battery module at different stages during the test.
Fig.2 Thermal runaway extension test curve of ternary lithium-ion battery module
Figure 3 Partial enlarged view of the thermal runaway extension test curve of the ternary lithium-ion battery module
Figure 4 Photo of thermal runaway expansion test of ternary lithium-ion battery module
Thermal runaway expansion of lithium iron phosphate lithium-ion battery modules
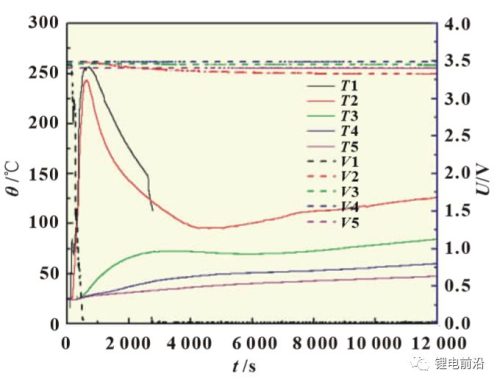
Figure 5 Thermal runaway extension test curve of lithium iron phosphate lithium-ion battery module
Figure 6 Partial enlargement of the thermal runaway extension test curve of the lithium iron phosphate lithium-ion battery module
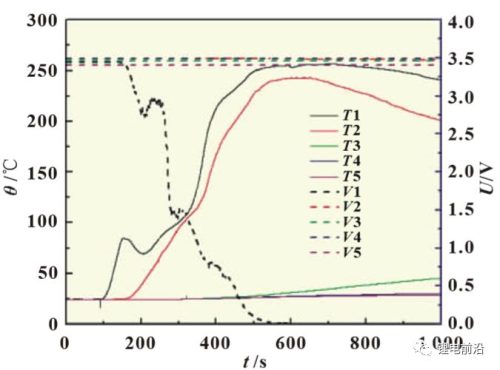
Figures 5 and 6 are the temperature and voltage curves of the thermal runaway extension test of the lithium iron phosphate lithium-ion battery module. It can be seen from the figure that with the heating, the 1# battery soon experienced thermal runaway, but after the 2#, 3#, 4# and 5# batteries were heated for more than 10,000s, only the temperature changed. A certain amount of rise, and no thermal runaway occurred. As can be seen from Figure 6, in the early stage of heating, the temperature of T1 quickly rose to about 85°C, and the voltage of the battery dropped at this time, from 3.45V to about 2.8V, and then slightly recovered. At the same time, T2 segment starts to rise. As the heating continued, the voltage of the 1# battery continued to drop, T1 and T2 continued to rise, and finally the voltage of the 1# battery dropped to 0V and a complete thermal runaway occurred, and the maximum temperature of T1 reached about 250°C. In the early stage of heating, when T1 reaches 80 °C, the battery has a voltage drop, which is mainly caused by the local leakage and short circuit of the battery due to the high temperature of the heating sheet used.
The specific heat capacity of the ternary lithium-ion battery and the lithium iron phosphate lithium-ion battery used in the test are basically the same. In the thermal runaway extension test, after the ternary lithium-ion battery module triggered thermal runaway of one battery, the other batteries suffered thermal runaway in turn, and showed a certain regularity in the development of thermal runaway; lithium iron phosphate The thermal runaway expansion of the ion battery module did not occur. After triggering a thermal runaway of one battery, the remaining batteries did not suffer from thermal runaway, and no thermal runaway occurred after 3 hours of continuous heating. Ternary lithium-ion batteries catch fire and burn violently during thermal runaway, releasing more energy than lithium iron phosphate lithium-ion batteries.