Research progress of anode materials for sodium ion batteries In the 1970s, research on sodium ion batteries and lithium ion batteries was carried out almost simultaneously. Later, due to the successful commercialization of lithium ion batteries, the research on sodium ion batteries stagnated. Until 2010, with the large demand for renewable energy utilization and the urgent need for large-scale energy storage technology, sodium-ion battery once again ushered in its golden period of development.
The principle of sodium-ion battery is similar to that of lithium-ion battery. The anode materials can also be divided into intercalation reaction materials, conversion reaction materials, and alloy reaction materials according to the sodium storage mechanism.
1. Embedding the reactive material
The intercalation reaction materials are mainly carbon-based materials, including graphite, nano-carbon materials, soft carbon and hard carbon materials.
Graphite anode material
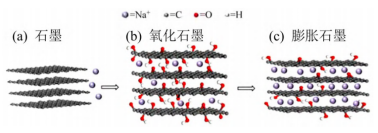
Graphite is a commercialized negative electrode material for lithium-ion batteries. However, compared with the ionic radius of Na+, the interlayer spacing of graphite is too small, so that Na+ is difficult to intercalate between graphite layers, even if Na+ migrates between layers after successful intercalation. Also very difficult. More seriously, Na+ reacts with graphite to form NaC64 compound, and the corresponding reversible capacity is only about 35mAh/g. In order to solve the above problems, the researchers tried to increase the interlayer spacing of graphite so that Na+ can easily intercalate between the graphite layers. They obtained expanded graphite by oxidizing graphite and used it as a negative electrode material for sodium-ion batteries. It was found that the expanded graphite exhibited good sodium storage performance and cycle stability (the capacity remained at 184mAh/g after 2000 cycles).
Soft carbon/hard carbon anode materials
Hard carbon and soft carbon materials are considered to be the most promising anode materials for Na-ion batteries. This type of material does not have the structural characteristics of graphitization, and its graphite crystallites are freely oriented, that is, short-range order and long-range disorder in structure. At the same time, the structure contains a large number of defects, which is very conducive to storing Na+ with a large ionic radius, so its sodium storage capacity is much larger than that of graphite. In addition, the use of soft carbon and hard carbon to construct composite materials is also one of the important development directions.
New Nanocarbon Materials
Since the 1960s, new carbon materials including carbon nanotubes and graphene have been developed. Carbon nanotubes have a unique one-dimensional tubular structure with a large aspect ratio; graphene (redox graphene) has an ultra-thin two-dimensional sheet structure, which is beneficial to ease the volume change during charging and discharging.
Using carbon nanotubes or graphene alone as the negative electrode of sodium-ion batteries can achieve good cycle stability, but its relative specific capacity is low. In recent years, the preparation of high-performance anode materials by combining graphene or carbon nanotubes with other alloy or conversion materials has become a hot research field.
2. Conversion reaction materials
The conversion reaction materials are mainly compounds composed of transition metals and oxygen, sulfur, selenium and phosphorus. Different from graphite, the above compounds show a certain sodium storage activity when used as anode materials for sodium ion batteries. The reaction principle is to react with Na+ to generate transition metals and corresponding sodium oxide, sodium sulfide, sodium selenide and sodium phosphide.
However, due to the high electronegativity of O, its chemical bonds with transition metals or Na+ are very stable, and the Na2O generated by the reaction is difficult to be completely reversible in the electrochemical process, so transition metal oxides also exist in sodium-ion batteries. Problems with voltage hysteresis and low coulombic efficiency. To this end, most researchers focus on transition metal sulfur/selenides. On the one hand, S and Se have slightly better conductivity and lower electronegativity than O, and Na2S and Na2Se can be more reversibly transformed during the reaction, thereby improving the Coulombic efficiency; on the other hand, transition metal sulfur/selenide is layered material, and the interlayer spacing is large, Na+ can be easily inserted, extracted and migrated between the layers, so good electrochemical performance can be achieved.
Although the conversion reaction material exhibits a large theoretical capacity, the volume expansion occurs during the sodium storage process, resulting in a low coulombic efficiency in the first week. At this stage, structural nanodesign, carbon-based material composite and element doping methods are usually used for conversion reaction materials to improve their electrochemical performance.