Compared with traditional lithium-ion batteries, the theoretical energy density of lithium-sulfur batteries can reach 2680Wh/kg under the condition of an average voltage of 2.15V, which is about 6 times the energy density of current lithium-ion batteries, so it is considered to be the most potential replacement. One of the systems of traditional lithium-ion batteries. In addition, compared with the expensive cathode materials of traditional Li-ion batteries, sulfur reserves are abundant, inexpensive, and environmentally friendly, which has unparalleled advantages in large-scale energy storage system applications.
How Lithium Sulfur Batteries Work
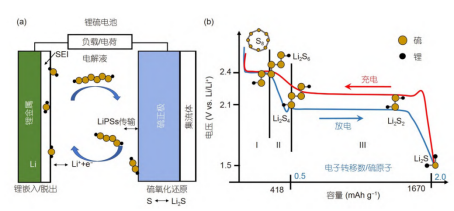
The structure diagram and typical charge-discharge curve of the lithium-sulfur battery are shown in the figure below. The negative electrode metal lithium is oxidized to release lithium ions and electrons, and at the same time, a protective layer SEI film is formed on the contact surface with the electrolyte; lithium ions and electrons move to the positive electrode through the electrolyte and external load respectively; elemental sulfur is reduced at the positive electrode to the discharge product lithium sulfide .
Schematic diagram of the structure of lithium-sulfur battery (a) and typical solid-liquid-solid charge-discharge curve of lithium-sulfur battery (b)
Elemental sulfur generally exists in the form of cyclic S8 in nature. During the discharge process, from solid cyclic S8 to liquid long-chain polysulfide Li2Sn (4<n≤8) and short-chain polysulfide Li2Sn (2<n≤4), and finally to solid discharge products Li2S2 and Li2S. There are two discharge platforms 2.3V (high platform) and 2.1V (low platform) on the discharge curve, in which long-chain polysulfides have higher solubility in the electrolyte, so the electrochemical reaction rate of this process is faster. , while Li2S2 and Li2S are almost insoluble in the electrolyte, and the reaction rate is slow. During the charging process, Li2S is directly oxidized to S8 through intermediate polysulfides, thus forming a complete and reversible redox reaction.
Challenges facing the commercial application of lithium-sulfur batteries
The invention of the lithium-sulfur battery dates back to the 1960s, when Herbert and Ulam filed a battery patent that first proposed the use of sulfur as an electrode material for energy storage devices. Today, the research work of lithium-sulfur batteries has been carried out for more than half a century, but due to its inherent defects, its commercial application still faces many difficult challenges:
①Dissolution and “shuttle effect” of LiPSs;
②Insulation of elemental sulfur and its discharge products;
③ The volume changes greatly;
④ Unstable SEI and safety issues caused by lithium negative electrode;
⑤ Low sulfur loading (area) or low sulfur content (%);
⑥ The ratio of electrolyte to active sulfur (E/S) is greater than 10 μL/mg in most cases, which hinders lithium-sulfur batteries from achieving high energy density;
⑦ Self-discharge phenomenon.
Lithium-sulfur battery defect improvement measures
In order to solve the above problems, research on lithium-sulfur batteries is usually carried out around the following aspects:
(1) The structural design of the sulfur composite cathode can effectively control the reaction path of the sulfide. Use one or more conductive/ion-conducting materials to composite with sulfur to build an electronic/ionic conductive network and uniformly disperse active sulfur into the host material to improve electrode conductivity and reduce polarization loss; choose a reasonable physical/chemical adsorption materials to effectively anchor LiPSs and prevent their “shuttle effect”; optimization and development of binders to stabilize the cathode structure, reduce the content of inactive substances and the amount of electrolyte, and improve the dispersion of active substances in the cathode and anchoring LiPSs, especially the development of functional binders such as ion/electronic conductivity; the design of novel current collectors and the construction of binder-free structures.
(2) Optimization of electrolyte. By selecting the salt components, solvents and new electrolyte additives that match the structure of the sulfur cathode, the electrolyte system is optimized, and the reaction kinetics and thermodynamic processes of sulfides can be effectively controlled at the micro and macro levels.
(3) Development and use of functional diaphragm/interlayer. In Li-S batteries, the used functional separator/interlayer can alleviate the diffusion of LiPSs to Li anode through physical blocking or chemical adsorption, regulate the transport behavior of Li ions, guide Li deposition, and achieve effective protection of Li anode. . In addition, the functional separator/interlayer can increase the conductive interface of the cathode, enhance the conversion kinetics of LiPSs, and improve the utilization of active materials.
(4) Protection of lithium negative electrode. LiPSs are oxidizing, and the strongly reducing Li metal can react with LiPSs shuttled to the negative electrode, resulting in severe corrosion of Li metal. Especially in the case of low E/S ratio and high sulfur loading, the high concentration gradient of LiPSs in the battery would exacerbate the erosion reaction. In addition, during repeated cycling, the SEI will be destroyed by the huge volume change, and the decomposition products of LiPSs will also become the components of the SEI, making the control of the SEI more complicated.