The electrode peeling strength of the lithium -ion battery has a significant impact on performance, so it is necessary to explore the influencing factors of the peeling strength. This article mainly discusses the impact of coating process and slurry.
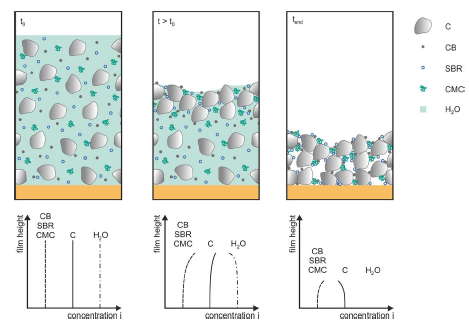
During the drying process, the internal structure of the electrode is changed
In the initial stage of drying, each component is evenly distributed in the electrode. During the drying process, due to the evaporation of the solvent and the contraction of the electrode membrane, there is a solubility gradient at the surface of the gas liquid, and the surface of the bending moon surface is formed on the surface of the solid particle layer. The solvent moved to the surface through the capillary force between the graphite particles and expands with the particle layer. The size of the SBR adhesive and charcoal black is at least 100 times smaller than graphite. The spacing of graphite particles is enough to allow SBR and charcoal black to flow freely. The solvent flow will move with polymers and small particles, making them rich in the upper layer of the electrode. After the solvent is completely evaporated, the electrode film shrinks.
The drying process is mainly divided into the following three stages:
(1) The content of the solvent at the air-membrane interface continues to decline until the critical concentration is reached.
(2) Starting from this critical point, the concentration of the solvent at the air-membrane interface remains unchanged,
(3) When the solvents evaporate further, the solvent of the air-membrane interface starts to decrease until the membrane is formed.
The effect of baking rate on peeling intensity
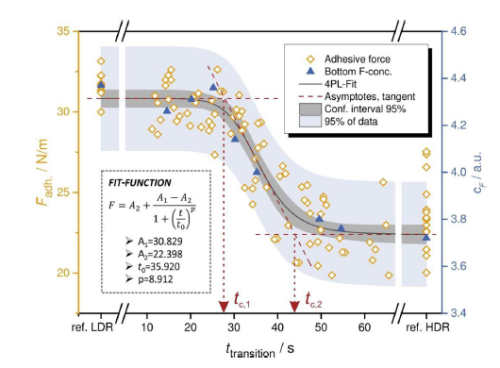
According to the analysis of the drying process, in the process of drying, the SBR adhesive and charcoal black move to the surface as the solvent evaporates. So how does the baking rate affect the distribution of the electrode internal component?
The following figure shows that under different baking rates, the concentration distribution of the bonding agent from the set flow to the qi and liquid interface, from the figure, the distribution of the bonding agent in different positions in the low baking rate (LDR) is relatively uniform. In the case of high baking rate (HDR), the concentration of adhesive concentrations from setting flow to gas -liquid interface continues to increase, and there is very little adhesive distribution in the flower and dressing area. In addition, the electrode impedance will increase accordingly.
The effect of coating surface density on peeling strength
If the fixing speed of the particles in the electrode structure is faster than the compensation flow speed of the solvent towards the electrode surface, the migration phenomenon of the adhesive will be greatly reduced. The smaller the solvent in the wet coating, the higher the temperature, and the faster the evaporation speed of the solvent.
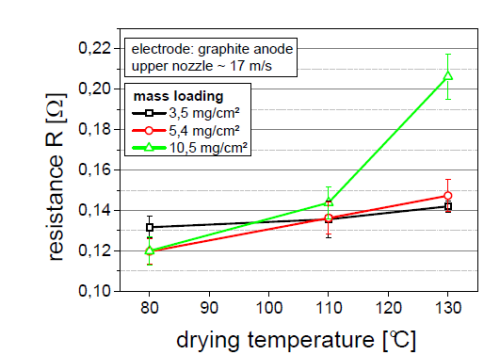
As can be seen from the figure below:
The low -surface density electrode, the electrode impedance is basically not affected by the temperature. This is mainly because the total amount of the solvent is low and the electrode structure is fixed, and the adhesive will not have obvious migration.
The high -surface density electrode has a completely different characteristic. The constant speed evaporation stage is very long, which provides sufficient time for the migration of the bonding agent. As the temperature rises, the phenomenon of bonding and carbon black migration will increase Poor and electrode impedance will increase.
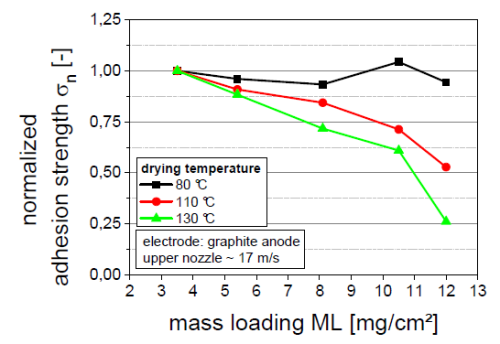
At 80 ° C, the peeling strength of different surface density is not much different.
When it reaches 110 ° C, the mobility speed will increase, and the surface density will increase to 5.4mg/cm2, then the drying time will increase, which will provide sufficient migration time for the bonding agent. The face density will be further increased to 12mg/// At CM2, the peeling intensity will be reduced by 52%.
When the temperature increases to 130 ° C, the migration phenomenon will further intensify, resulting in poor peeling strength.