All-solid-state batteries (ASSBs) using inorganic solid electrolytes (SEs) have been the focus of next-generation energy storage devices due to their potential for excellent safety and high energy density. Recent advances in this field are mainly based on material advancements, such as the discovery of SEs with high ionic conductivity and improved interfacial stability of electrodes. However, the use of inelastic SE leads to severe electrochemical-mechanical failures, such as cathodic active material (CAM) disintegration, CAM/SE contact loss, and stress accumulation during cycling, deteriorating the Li+ and e- transport pathways.
The mechanical properties of SEs are a key factor in the development of practical all-solid-state batteries (ASSBs), as they affect the effective contact area between SEs and active materials, and in turn, the fabrication methods of electrodes. For example, the application of “brittle” oxide SEs has been very complicated because they cannot make good ionic contact with CAMs without the help of liquid or polymer electrolytes. In contrast, thick composite electrodes with high mass loadings can be fabricated by employing plastic SEs (i.e., sulfides and polymers), providing high initial areal capacities comparable to their LE counterparts. However, the intrinsic properties of inorganic solids, namely transfer and absorption of external stress, have important electrochemical-mechanical effects on the performance of ASSBs.
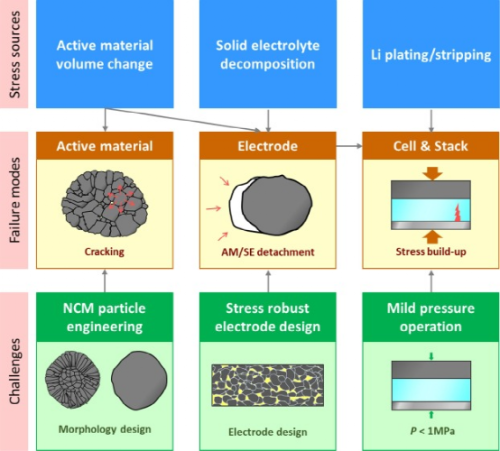
Figure 1. Classification of stressors, possible chemical-mechanical failure modes, and challenges to ASSB.
Electrochemical-mechanical effects in ASSB are considered to be a multiscale topic. Related issues are categorized in Figure 1. Clearly, the actual behavior of ASSBs is more complex than what we discuss here because of the irregular distribution of SEs and pores in the cell. The main stressor of ASSBs is the volume change of the active material upon cycling. First, this creates internal microcracks within the CAM particles. The accumulation of these microcracks eventually leads to the “disintegration of the CAM particles. Even at high external pressures of tens of MPa (MPa), the internal cracks are not absorbed by SE. This is in stark contrast to LIB, where LEs penetrate into the cracks. , and upon cycling, the active contact area increases.
Second, the repeated volume expansion/contraction of the active material cannot be easily accommodated by the deformation of the SE, which leads to the “detachment” of the SE from the active material, and thus the effective contact area of the CAM/SE is reduced.
Furthermore, in a recent study, careful observation of the initial charging phase revealed that the redox reaction of SEs induces local stress in the composite electrode. These two stressors, i.e., active materials and SEs, jointly caused the electrochemical-mechanical failure of the composite electrode.
Battery requirements for solid electrolytes
For advanced ASSB technology, solid electrolytes should meet multiple requirements, including high ionic conductivity, wide electrochemical stability window, chemical stability to ambient air, thermal stability, and mechanical properties.
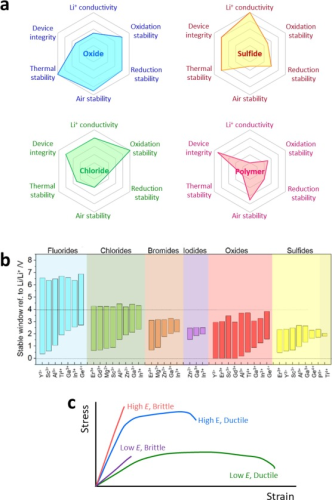
Figure 2. (a) Comparison of various SE compounds for ASSB application requirements. (b) Comparison of the electrochemical stability windows of inorganic SEs. The upper and lower limit potentials were estimated by DFT calculations. (c) Typical stress-strain curves for brittle and ductile materials.
Figure 2a compares the performance characteristics of four main SEs: oxides, sulfides, chlorides, and polymer electrolytes. Among them, high ionic conductivity comparable to LEs is a prerequisite for operable ASSBs at room temperature. The oxide SE exhibits moderate ionic conductivity at room temperature, approximately 10-6-10-3 S cm-1. Solid polymer electrolytes (SPEs), in which lithium salts are dissolved into the polymer matrix, show very low ionic conductivity (10-8-10-7 S cm-1 at room temperature). The addition of oxide ceramics to SPE to form composite polymer electrolytes (CPEs) can significantly improve the ionic conductivity, which can be attributed to the reduced crystallinity of polymer domains, interfacial conduction effects, and the contribution of oxide SEs. However, their maximum ionic conductivities are two orders of magnitude lower than LEs (∼10-2 S cm-1). In contrast, recent searches for sulfide and halide SEs have yielded high ionic conductivities, reaching maxima around 10-2 and 10-3 S cm-1, respectively.
The chemical stability of SE under humid conditions is important for reliable and cost-effective battery production. Although oxide SEs are not affected by complete hydrolysis, their surface degradation severely hinders the interfacial transfer of Li+. Sulfide SE is highly sensitive to humidity and produces toxic H2S gas.
The feasible operating voltage of ASSB is constrained by the electrochemical stability of SE. Polyethylene oxide (PEO)-based SPEs start to be oxidized at 4.0V (vs. Li/Li+), which leads to high interfacial resistance when combined with 4V-grade CAMs such as LiCoO2. This interfacial degradation problem is solved by introducing surface coatings such as Li3PO4, Al2O3, and poly(ethylcyanoacrlate) (PECA). It is predicted that the electrochemical oxidation stability of inorganic materials is determined by the anion species, indicating that the anion is the center of oxidative decomposition (Fig. 2b). From the voltage upper limit, the order of intrinsic electrochemical oxidation stability is: fluoride > chloride > bromide ≅ oxide > sulfide ≅ iodide. Sulfide SEs show a very narrow stability limit, around 2.5 V (vs. Li/Li+). The oxidative stability of oxides is mainly affected by metal ions. Importantly, the chloride SE is predicted to be stable to 4.3V (vs. Li/Li+), which renders the protective layer on the 4V-level layered oxide CAM non-existent. Notably, fluoride exhibits particularly high oxidative stability (>6 V vs. Li/Li+), which is offset by its low ionic conductivity. This is due to the small ionic size and high electronegativity (and thus low polarizability) of F-. Importantly, the electrochemical decomposition of SE has been extensively studied with the formation of resistive decomposition products at the interface, which explains the actual electrochemical window.
In summary, focusing on the use of inorganic SEs, a hierarchical review of the mechanical properties of SEs, sources of internal stress generation in ASSBs, and their electro-mechanical effects on the various components of ASSBs is performed.
Despite the ductility of sulfide or halide SEs, their inelasticity cannot fully comply with the evolution of internal stress, resulting in some electrochemical-mechanical failures such as detachment of CAM/SE, isolation of CAM primary particles, and challenge to stacking pressure , these failures are much more complex than those discussed in this article.
In addition to materials engineering, the interaction between external and internal stress and the challenges of mild pressure operation are discussed.
We propose the search for novel CAMs with more mechanical compliance and electrode engineering, such as the optimization of binder/carbon, as an important topic for future research. Such solutions could impart elastic mechanical properties to composite electrodes and mitigate electrochemical mechanical failures. Besides the isostatic pressing technique, a feasible large-scale fabrication method of ASSBs using inorganic SEs is also essential for commercialization, where the mechanical properties of electrodes and separators are important parameters.
Overall, despite some recent achievements in SE materials, protective layers, and electrode optimization in ASSBs, understanding the electrochemical-mechanical behavior in ASSBs and achieving state-of-the-art ASSB performance under realistic operating conditions will be a daunting task. challenge.