Lithium-ion battery is the most common chemical energy storage power source in our life, and its safety is our eternal concern. In order to improve the safety of lithium-ion batteries, a battery control circuit (BMS) has been added to control the charging and discharging of the batteries to prevent safety risks caused by overcharging and overdischarging of lithium-ion batteries.
In the structural design of lithium-ion batteries, three-layer composite separators and ceramic-coated separators are used to improve the safety of lithium-ion batteries under high temperature conditions.
However, there is still a type of safety risk that is unavoidable even if a complete safety design is made. This is the thermal runaway of lithium-ion batteries caused by mechanical abuse. For example, when the lithium-ion battery is subjected to external mechanical pressure, the battery is deformed or punctured, causing The positive and negative electrodes are short-circuited, and the power of the entire lithium-ion battery is released in a short period of time through the short-circuit point, which will generate extremely high temperatures at the short-circuit point, resulting in the decomposition of the positive electrode active material and the release of extremely strong free oxygen in the oxidizing machine for further oxidation. The electrolyte, which generates a large amount of heat, eventually leads to thermal runaway of the lithium-ion battery, causing fires and explosions.
More seriously, if the thermal runaway occurs on a battery in the battery pack, the high temperature released by the thermal runaway battery will cause the thermal runaway to spread inside the battery pack, causing serious consequences.
Therefore, how to avoid thermal runaway in lithium-ion batteries and how to suppress the spread of thermal runaway inside the battery pack has become the focus of attention. Phase Change Composite PCC for Inhibiting Thermal Runaway Propagation within a Battery Pack.
What I want to introduce to you today is a thermal runaway inhibitor added to the battery. The main function of this material is that when the lithium-ion battery is mechanically abused, it can be released in time inside the battery, thereby inhibiting thermal runaway. develop.
The study shows that by adding 4% of the inhibitor to the battery, the maximum temperature of the battery in the puncture test can be reduced by 50%, and the material has little effect on the cycle performance of the battery. The research results were recently conducted by the University of California, San Diego. Yang Shi et al. published in the journal JPS.
Generally speaking, although some traditional electrolyte flame retardants can increase the safety of lithium-ion batteries, they will seriously reduce the cycle performance of lithium-ion batteries. In order to solve this problem, Yang Shi et al. The thermal runaway inhibitor DBA (dibenzylamine) is wrapped in a capsule structure and placed inside the battery. When the battery receives external mechanical pressure, the capsule structure will be destroyed and the thermal runaway inhibitor will be released. Inhibit the occurrence of thermal runaway.
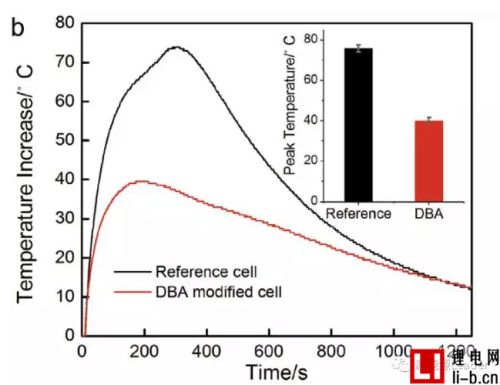
In the test, the LIR-2450 button battery was used for testing. In the acupuncture test, the temperature rise of the experimental group battery with DBA was 40 degrees Celsius, while the temperature rise of the control group test battery reached 75 degrees Celsius. The battery temperature rise is reduced by about 50% in thermal runaway caused by mechanical abuse.
Through calculation, it can be found that the heat released by the experimental battery during thermal runaway is 0.15Wh, while the control battery released 0.23Wh of heat during thermal runaway. By adding 4% DBA to the battery, the battery is in thermal runaway. The heat generation of the battery is reduced by about 1/3.
In the extrusion test, Yang Shi put 5% DBA of the battery cell weight into an aluminum-plastic film bag and put it into the battery. When the battery was deformed by extrusion, the aluminum-plastic film ruptured and the DBA was released into the electrolyte. , the test results show that by adding DBA inhibitors to the battery, the battery temperature rise is reduced by about 50% during the thermal runaway caused by extrusion, which is consistent with the results of the acupuncture test.
In order to reveal the working principle of DBA in lithium-ion batteries, Yang Shi also studied the reactivity between DBA and the positive and negative electrodes. It was found that DBA can react with the fully charged positive electrode to form a solid-electrolyte film on the surface of the positive electrode. The resistance of charge exchange increases.
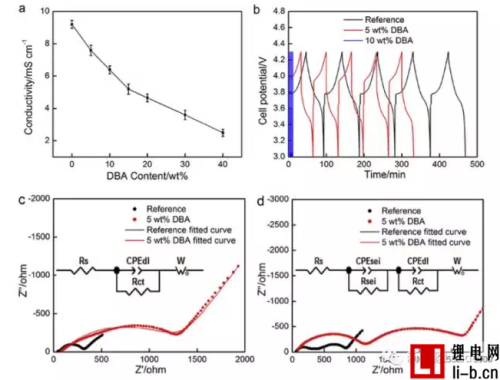
The ionic conductivity test of the electrolyte found that the addition of DBA greatly reduced the conductivity of the electrolyte. The conductivity of the pure electrolyte was 9.23 mS/cm. After adding 5% and 10% of DBA to the electrolyte, electrolysis The resistivity of the liquid dropped to 7.59 mS/cm and 6.38 mS/cm. Test the migration number of Li+ in the electrolyte. In the control group, the migration number of Li+ is 0.48, while the electrolyte of the experimental group with 5% DBA added, the migration number of Li+ is only 0.23.
From the above analysis results, it can be seen that by adding DBA to the electrolyte, the charge exchange resistance increases, the ionic conductivity of the electrolyte decreases, and the Li+ migration number decreases. In general, the migration of Li+ between the positive and negative electrodes is inhibited. Thereby reducing heat generation.