With the implementation of environmental protection laws and the macro-control of policies, the demand for electric vehicles is increasing year by year. Due to its own series of advantages, lithium-ion batteries have gradually extended from the field of consumer electronics to other industrial production fields, such as large-scale energy storage, automotive industry, marine power supply, etc. In order to meet consumers’ requirements for electric vehicle range, the development of high-energy-density lithium-ion batteries has received great attention. It is worth noting that the specific energy and stability of cathode materials are the main obstacles hindering the development of high-energy-density Li-ion batteries. Layered Li[NixCoyMn1-x-y]O2 (NCM) and Li[NixCoyAl1-x-y]O2 (NCA) have relatively low cost, superior reversible capacity and rate performance, so they are promising high-energy-density lithium ions battery cathode material.
The researchers proposed to increase the energy density of the material by increasing the Ni content in the layered ternary cathode material. Cathode materials with a Ni content of more than 83% can meet customers’ mileage requirements of more than 300 miles in the field of electric vehicles. However, too high Ni content in the material will destroy the structural stability of the material. The reaction of the highly active Ni4+ generated during the charging process with the electrolyte will generate a NiO-like rock-salt phase, which seriously damages the structure of the layered material, leading to the collapse of the cathode structure, which in turn induces the dissolution of transition metal ions, phase transformation, and lattice oxygen precipitation. Among them, the change of lattice parameters of high-nickel cathode materials during the charging and discharging process will lead to the formation of microcracks. The resulting microcracks expose new surfaces inside the particles, further accelerating structural decay. It is worth noting that the higher the nickel content, especially when the nickel content exceeds 80%, the more obvious the damage effect of cracks is. To sum up, the main reason for the decreased cycle life of high-nickel cathode materials is microcracks, which can reduce the thermal stability, structural stability and cycle stability of cathode materials at the same time
1. The mechanism of crack generation
Cracks can be divided into two types: intergranular cracks and intragranular cracks, both of which can damage the structure of the cathode material. This section mainly summarizes the characteristics, influencing factors and generation mechanisms of these two types of cracks.
1.1 Generation mechanism of intergranular cracks
Intergranular cracks are mainly caused by the anisotropic shrinkage and expansion of primary particles produced by the H2→H3 phase transition. Intergranular cracks are considered to be the most important degradation mechanism of high-nickel cathode materials (its impact on the material is much greater than that of intragranular cracks), and the specific reasons are as follows: (1) Intergranular cracks will destroy the contact between primary particles, resulting in The electrical conductivity inside the secondary particles decreases, so that the state of charge (SOC) inside the secondary particles is not uniform, and the non-uniform SOC will cause the material to shrink/expand unbalance, which in turn leads to the further development of cracks; (2) the appearance of cracks It exposes more active sites inside the particle to react with the electrolyte, creating new suitable conditions for phase transition, corrosion and side reactions, thereby accelerating the failure of the material.
1.1.1 Due to H2→H3 phase transition
The volume change caused by the phase transition has a much greater effect in the c-axis direction than in other directions. In fact, the stress inside the particle is not uniformly distributed, the stress is only distributed at certain points inside the particle. High nickel layered oxides are generally spherical secondary particles composed of primary particles. Among them, the primary particles are irregular polyhedrons, so the direction of the anisotropic deformation of the primary particles due to the phase transition is random. In addition, the arrangement of the primary particles is irregular, which allows the stress generated by the expansion and contraction of two adjacent primary particles to interact at the grain boundaries. Therefore, the characteristic secondary particle structure of high-nickel cathodes leads to stress concentration at the grain boundaries during charge and discharge. This force acting on the grain boundary is the direct cause of the generation of intergranular cracks.
1.1.2 SOC heterogeneity among different primary particles causes cracks
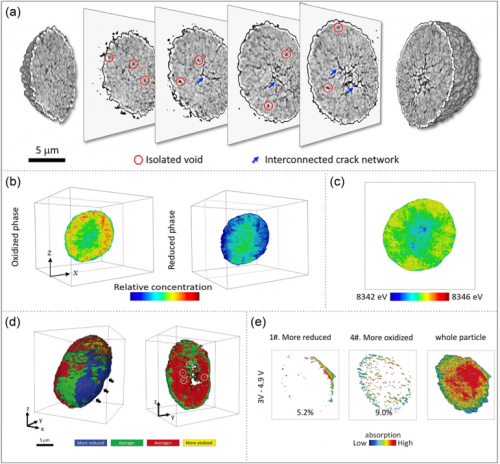
Inhomogeneous SOC can lead to the generation of oxygen vacancies and voids. The relationship between porosity and SOC stems from charge compensation. According to the charge compensation mechanism, in the extremely Li-poor region, Ni is oxidized and the 2P orbital of O is depleted. The electrolyte reduces the highly unstable Ni4+ to Ni2+, resulting in the transfer of electrons from O atoms to Ni. O loses electrons and the valence state rises and eventually evolves into oxygen, which is the reason for the formation of oxygen vacancies. When the oxygen is continuously oxidized, the active material generates gas and the porosity increases. It can be considered that there is a positive correlation between the porosity and the occurrence of cracks.
Besides, when the SOC is not uniform, it means that there is a Li-depleted region in the secondary particles. Due to the charge compensation effect, Ni2+ near Li vacancies will be oxidized to Ni4+. Since Ni and O also have a charge compensation effect, O vacancies will appear, which reduces the migration barrier of Ni2+ and makes it easier for Ni2+ to migrate to the Li layer, which will form a rock-salt phase region. Adjacent particles of different phases can create stress concentrations at the phase interface. This is because the adjacent primary particles with heterogeneous phase structure will produce different volume changes during the entire charge-discharge process. The layered structure region and the adjacent rock-salt structure region generate forces at the grain boundaries of the two primary grains, resulting in cracks along the grain boundaries at the grain boundaries.
Characterization of SOC heterogeneity
1.2 Generation mechanism of intragranular cracks
According to the crack generation mechanism and morphology, intragranular cracks can be divided into three categories: (1) classical cracks: in the layered structure, except for the tip, the two free surfaces are parallel to the entire crack direction and the (003) plane; (2) deep Color contrast fringes: corresponding to the widened TM interfacial spacing; (3) fine low-angle grain boundaries: Moiré fringes generated by optical interference can be observed due to the special arrangement morphology of such cracks.
1.2.1 Non-uniform lithium deintercalation inside the particles
To a certain extent, the deintercalation of Li ions reduces the stability of the structure and amplifies the intragranular strain. The non-uniform stress field inside the secondary particles and the non-uniform Li deintercalation together lead to the generation of classical cracks and dark contrasting fringes. In fact, a tensile stress field prevails in the extremely lithium-deficient region caused by the uneven deintercalation of lithium ions, and the tensile stress is the main stress for crack generation. In addition, the out-of-plane part of the layered structure is more prone to crack formation, since the Li-O bond is the main connection between the out-of-plane transition metal layers. Due to the inhomogeneous distribution of Li ions, the Li-O in the Li-poor region is reduced, which weakens the interlayer force, which causes serious structural damage. Besides, when the SOC is heterogeneous, the phase structure changes in the extremely Li-poor region due to the massive loss of Li-O bonds. This will lead to an imbalance of lattice parameters between different regions due to the inhomogeneity of the structure, which is also one of the causes of stress.
1.2.2 The effect of mechanical force inside the particle
1) Fatigue strain
The generation and closure of cracks during charging and discharging are caused by the expansion and contraction of primary particles when they are cycled over long periods of time. As the particles expand and contract repeatedly, low-angle grain boundaries appear inside the primary particles after a few cycles.
2) Anisotropy inside the primary particle
In the same primary particle, the orientation of the crystals is different. Therefore, this anisotropy of crystal orientation can induce stress concentration inside the crystal during cycling.
3) There is a “pseudograin boundary” inside the primary particle
The primary particles are not single crystals and have grain boundaries (GBs) inside them, and these intragranular GBs will eventually develop into intragranular nanocracks. Grain boundaries within the grain lead to anisotropic forces within the primary particles, which eventually develop small-angle cracks within the grain.