At present, the ternary cathode materials NCM111, NCM523 and NCM622 for lithium-ion batteries have been put into mass production. From the perspective of cathode materials, the increase of nickel content will lead to the intensification of Li/Ni mixing in the ternary material and shorten the cycle life.
More seriously, the increase of nickel content will lead to a significant increase in the residual alkaline impurities between particles, which will lead to serious gas production during the charging and discharging process, resulting in bulging deformation of the battery, shortened cycle and shelf life, and potential safety hazards. The residual alkaline impurities have become the key to restrict the application of high-nickel ternary materials in high-energy-density power batteries for electric vehicles.
In addition, in recent years, methods such as various anion and cation doping or coating have been adopted to stabilize the bulk phase structure of ternary materials and achieve the effect of improving cycling and storage performance. These methods are difficult to solve the problem of high residual alkali impurities in high nickel materials. To this end, the author of this paper studied the residual alkali impurities under different sintering temperatures and lithium/metal ratios when NCM811 materials were prepared by high-temperature solid-phase method, and verified the alkali reduction effect of various post-treatment systems.
1. Experiment
1. Synthesis of NCM811 material
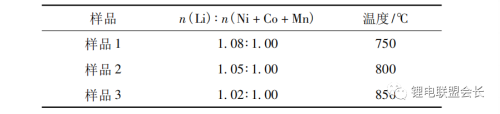
The NCM811 material precursor Ni0.8Co0.1Mn0.1(OH)2 and LiOH are mixed according to the set stoichiometric ratio. In a high-speed mixer, the raw materials were mixed at a speed of 700r/min for 0.5h. The mixture was placed in a corundum crucible, placed in a box-type atmosphere sintering furnace, sintered in an oxygen atmosphere and at a specific temperature for 12 hours, and cooled to room temperature naturally in an oxygen environment to obtain the NCM811 sample. Three test points were selected in different lithium ratios and temperature regions. The n(Li):n(Ni+Co+Mn) and sintering temperatures corresponding to each sample are shown in Table 1.
2. XRD and SEM analysis of samples
The structure of the sample was analyzed by powder X-ray diffractometer, CuKα, wavelength 0.15406nm, tube pressure 40kV, tube flow 40mA, scanning speed 2 (°)/min, step size 0.02°. The surface topography of the samples was analyzed by field emission scanning electron microscopy.
3. Analysis and treatment of residual alkaline impurities in samples
NCM811 (made in Japan), NCM622 (made in Hunan), NCM523 (made in Zhejiang) and NCM111 (made in Fujian) were selected as comparative samples for the analysis of basic impurities. Place 5g of powder sample in 95g of deionized aqueous solution, stir for 5min and then suction filter. Use a potentiometric titrator to calculate the contents of Li2CO3 and LiOH in the solution according to the equivalent point values V1 and V2, and use the contents as impurities to represent the composition.
According to the content of Li2CO3 and LiOH in the measured sample 2, the above-mentioned basic impurities of 20%, 50% and 80% of the theoretical value were consumed as the end points, and the alkali-reducing substance ammonium dihydrogen phosphate was calculated and added. After fully reacting and evaporating to dryness with constant stirring, backfired in an oxygen environment of 700° C. for 5 h to obtain sample 2-P2, sample 2-P5, and sample 2-P8. In addition, with the solid-liquid mass ratio of 1:4, the comparative sample 2-H2O was prepared by rinsing with pure water, and the backburning conditions were the same.
4. Positive pole piece fabrication and simulated battery assembly
The NCM811 sample, polyvinylidene fluoride, and acetylene black were mixed in a mass ratio of 92:5:3, and after grinding uniformly, coated on a 0.1 mm thick aluminum foil, and punched into a circular positive plate with a diameter of about 14 mm, which contained about 10 mg The positive electrode material was finally vacuum-dried at 120 °C for 12 h. A CR2032 button cell was assembled in an argon-protected glove box with lithium metal sheet as the negative electrode, Celgard 2325 membrane as the separator, and 1 mol/L LiPF6/EC+DMC (mass ratio 1:1) as the electrolyte.
5. Electrochemical performance test
The charge-discharge test was carried out at 22°C with CT4008 battery performance test system. Rate performance test: at 3.00-4.30V, cycle at 0.10C, 0.20C, 0.50C, 1.00C, 2.00C and 5.00C in turn, and compare the discharge specific capacity with the value at 0.01C. Cycle performance test: first charge to 4.3V with 1.00C constant current, turn to constant voltage to charge until the current is 0.01C; then discharge with 1.00C constant current to 3.0V.
2. Results and discussion
1. XRD analysis of sintered material
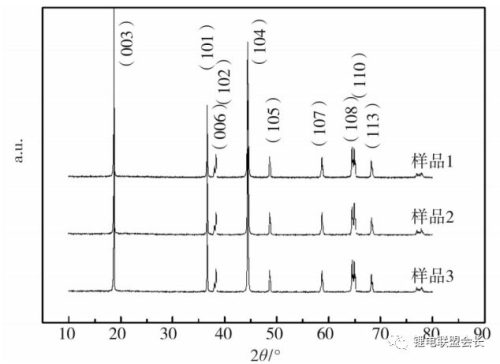
Figure 1 is the XRD pattern of the prepared NCM811 material. As can be seen from Figure 1, NCM811 prepared under different temperature and lithium ratio conditions did not appear impurity peaks, and each sample was of α-NaFeO2 structure. The degree of splitting of the (006)/(102) and (108)/(110) crystal planes can usually be used to measure the degree of order of the layered two-dimensional structure.
3. Conclusion
In this paper, the author prepared a high-nickel ternary cathode material NCM811 through a high-temperature solid-phase synthesis process. The product is α-NaFeO2 structure without impurity phase. Using the sintering conditions of low lithium ratio and high sintering temperature, the residual basic impurity content of the sample is still higher than that of NCM523, NCM111 and other low nickel content ternary materials, indicating that high basic impurities are the common characteristics of high nickel ternary materials.
In a liquid phase environment, NCM811 was treated with different amounts of ammonium dihydrogen phosphate and secondary sintered. The results show that the residual alkali content on the surface of the material is reduced, and the crystal structure does not change, but electrochemically inert substances remain on the surface of the particles, resulting in a significant decrease in capacity and cycle retention. This shows that the idea of reducing residual alkaline impurities by conversion is feasible, but it is necessary to further optimize the amount of phosphate added and the temperature of backburning.
Using water washing can greatly reduce the content of alkaline impurities, and it is lower than the level of imported products. Compared with the sample before treatment, the specific capacity increased by about 1.5mAh/g, and the capacity retention rate after 100 cycles also increased from 90.8% before treatment to 94.1%.
The above results show that water washing is a convenient and effective method to control the content of alkaline impurities in high-nickel ternary materials and improve the properties of materials. As the basic process of controlling residual alkali in high nickel ternary materials, water washing can exert the performance of the material itself. The follow-up needs to focus on how to combine water washing and alkali reduction with coating, so as to directly convert the residual basic impurities of alkaline impurities into the coating layer that can realize Li+ conduction on the surface of the primary particles of the positive electrode material, so as to further improve the utilization rate of lithium resources and at the same time. Improve the electrochemical performance of cathode material products such as cycle and thermal stability.