In 2012, Amartya Mukhopadhyay of Brown University of Engineering and Technology adopted an optical stress sensing device to measure stress in thin films in real time (in situ) to study the development of irreversible stress in graphite electrodes during cycling of lithium-ion batteries. The author experimentally explored the electrochemical behavior of the initial cycle of the graphite electrode, and deeply analyzed the evolution of the irreversible stress of the electrode in this process from the perspective of electrochemical, cycle times, electrode thickness, voltage, etc. change), which provides a new idea for the study of the actual mechanical properties of lithium-ion batteries.
Focusing a parallel laser beam on the backside of the quartz substrate (graphite electrode carrier), the rigid substrate limits the expansion and contraction of the in-plane dimensions of the active thin film (here graphitic carbon) during lithium intercalation/delithiation, so that the substrate / The thin film system bends and deflects the laser beam as it reflects off the substrate. Since the quartz substrate undergoes elastic deformation, the stress in the thin film is proportional to the induced change in the wafer curvature, and by monitoring the change in the spot spacing of the deflected beam reflected from the backside of the substrate, in-situ measurement of the stress development in the graphite electrode can be achieved.
Sample: Graphitic carbon film (CVD C), a graphitic carbon layer prepared by chemical vapor deposition (CVD) and graphitization principles on a 250 nm thick quartz wafer with a diameter of 1 inch; and a graphitic carbon layer by atomic layer deposition (ALD) A 0.5 nm-thick layer of Al2O3 was deposited to block the formation of SEI and intercalation of solvated ions on the anode material to study its effect on the electrochemical behavior and consequent stress.
Experiment content: Assemble a model cell of CVD C film on Li metal in a custom electrochemical cell, perform galvanostatic discharge/charge cycles, and study the electrochemistry of CVD C film by monitoring the curvature of the substrate (bending of the substrate/thin film system). behavior and consequent stress changes.
Result analysis
1. Electrochemical behavior in the initial cycle
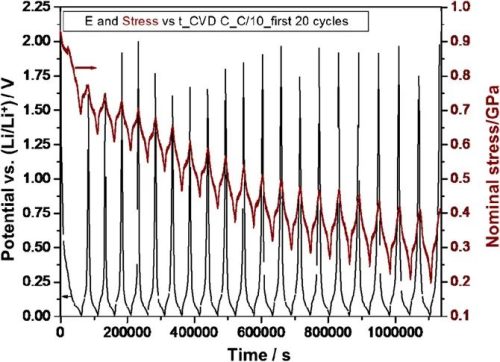
Figure 1. Potential and stress versus time for CVD C lithium intercalationdelithiation cycles
In the first cycle, a huge Li intercalation capacity was observed during the Li intercalation half-cycle, while the reversibility was relatively small during the de-Li half-cycle, except that after some apparent irreversible capacity loss occurred in the first cycle, the other cycles Most capacities are reversible and consistent for different cycling rates. At the electrochemical cycling rates of C/5, C/10 and C/20, the graphite thin film electrode (CVD C) was repeatedly charged with Li to close to the theoretical capacity. limit. Furthermore, after 50 cycles, there is no capacity fading or any apparent macro- and micro-structural damage, and the voltage data are flat.
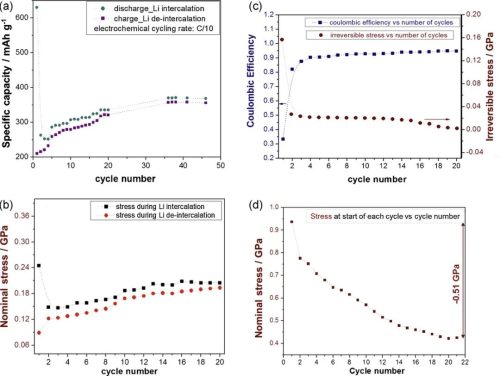
Figure 2. (a) Li capacity as a function of cycle number during half-cycles of intercalation and delithiation; (b) Nominal stress measured during half-cycles of intercalation and delithiation; (c) Coulombic efficiency and irreversible stress; (d) the stress present in the sample at the beginning of each cycle;
The irreversible capacity, i.e. the difference in Li capacity during the intercalation/delithiation process, is described by the Coulombic Efficiency (CE). It is mentioned in the article that the cumulative irreversible stress in the initial cycle is 2 times higher than the stress induced by the reversible stress, and the surface effect of the irreversible stress (film thickness effect) shows that the irreversible component is mainly caused by one or more phenomenon determines. In the first cycle, high capacity and consequent high stress were recorded during the intercalation half-cycle, with relatively low capacity and stress reversals during delithiation, resulting in capacity and stress during the first two half-cycles There is a large difference between the Coulombic Efficiency (CE) values in the first cycle, which are very low. The correlation of the electrochemical behavior with the stress development in the first 20 cycles and the fact that the irreversible stress is only from the surface phenomenon suggest that a large amount of irreversible stress is directly related to the formation of the SEI layer.
The stress evolution during the initial cycle is also shown in more detail in Figures 2b–2d, where the authors designed a graphitic carbon film with lattice coefficients parallel to the substrate so that the stress development is only related to the change in this film. During discharge (lithiation), a net compressive stress develops, which is then reversed upon charging (delithiation). A large fraction of the compressive stress is not reversed during the first lithium intercalation cycle. The magnitude of the irreversible stress decreases significantly in the second cycle, and this trend continues until the difference between the stresses in the later two half cycles is negligible, i.e. the stress development during delithiation/intercalation is almost completely reversed . The authors argue that this irreversible stress is an important feedback component for evaluating material properties.
In this study, an optical stress sensing device was used to measure the stress in thin films in real-time (in situ), and for the first time, the huge irreversible effects of graphite electrodes against Li metal were reported during the first few electrochemical cycles of graphite electrodes against Li metal. Evolution of compressive stress. The relationship between the irreversible stress and the formation of SEI was determined from multiple perspectives, which provided important guidance for the study of the electrochemical behavior and actual mechanical properties of lithium-ion batteries.