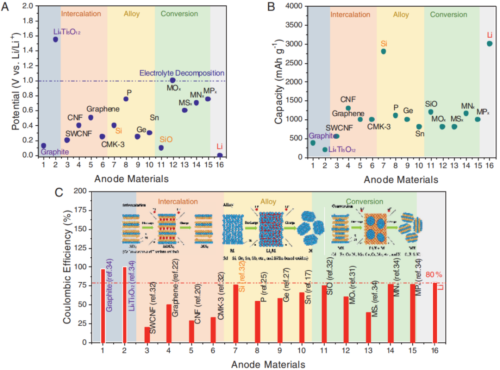
The high initial ALL of the anode occurs in the first few cycles with low coulombic efficiency (CE < 100%), which indicates that some Li+ remains in the anode, leading to a decrease in the number of cyclable Li+ in LIBs. When matched with the cathode, the reduced cyclable Li+ will inevitably lead to lower energy density of the whole battery. Figure 1 shows the typical intercalation/insertion, conversion, and alloyed lithium storage mechanisms for anode materials, which mainly exhibit relatively low potentials and have much higher capacities than commercial graphite and Li4Ti5O12, but the first laps of these materials The coulombic efficiency is usually lower than 80%, which leads to the mechanism of low coulombic efficiency. The reasons for the initial ALL of the negative electrode can usually be divided into the formation of SEI, the loss of active material materials, and the appearance of dead lithium.
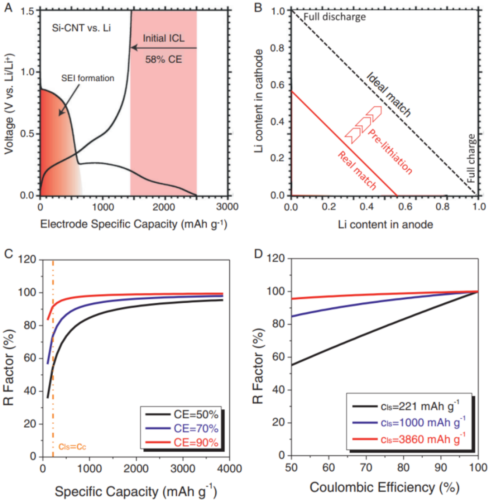
The initial ALL is caused by an irreversible electrochemical process on the negative electrode, so the most direct strategy to eliminate the initial ALL is to prepare a pre-lithium-intercalated negative electrode by electrochemical and/or chemical strategies before pairing with the positive electrode. The strategies for the cathode can be divided into three categories: half-cell electrochemical method (HC-EM), short-circuit electrochemical method (SC-EM) and chemical method (CM) as shown in Fig. 3. After the negative electrode is pre-inserted with lithium, the problem of large initial ALL can be well solved, and the Coulomb efficiency of the entire battery can be effectively improved in the first cycle.
HC-EM, a prelithiation strategy widely used in laboratory studies, can be achieved by constructing a half-cell structure consisting of the desired anode material as the cathode and Li metal as the anode. After the pre-lithium insertion is completed, the pre-lithium negative electrode is removed from the half-cell and reassembled with the positive electrode to form a full cell. The most important advantage of this strategy is the simplicity of the laboratory scale, which can be controlled by current as well as voltage termination after pre-lithium intercalation.
In order to avoid the complicated operation and extensive use of electrolytes caused by HC-EM, a strategy of directly contacting the negative electrode with lithium metal foil is proposed. Compared with HC-EM, SC-EM can not only generate SEI with very similar properties on the surface during the pre-lithium intercalation process, but also without sacrificing the structural stability of the anode.
3.3 CM
Various chemical approaches have been proposed in recent years to directly produce pre-lithium materials as anode candidates to match the cathode, and the pre-lithium results are more stable than products from electrochemical pre-lithium strategies. However, it is very difficult for the electrode preparation of the pre-lithium-intercalated negative electrode material obtained by this method. Due to the high chemical activity of the pre-lithium-intercalated negative electrode material, an anhydrous solvent must be used and carried out in a dry atmosphere. Similar to HC-EM and SC-EM, CM still requires a dry atmosphere to assemble the whole cell due to the unstable chemistry of the prelithiated anode. Therefore, the methods of adding lithium sources into the negative electrode all face the challenge of harsh conditions, which will inevitably increase the manufacturing cost.
【Prospect】
On the basis of summarizing common prelithiation techniques, this paper re-evaluates the importance of prelithiation strategies for next-generation LIBs. Since the current prelithiation strategies have multiple bottlenecks in practical applications, the author summarizes some possible breakthroughs the direction of:
4.1 Lithium source added to the negative electrode
Lithium sources can be preloaded into the anode by three methods, namely HC-EM, DC-EM and CM. HC-EM is an efficient method to utilize half-cells to prepare pre-lithium anodes paired with cathodes for full-cell electrochemical evaluation. DC-EM can also play the same role as HC-EM. Compared with these two strategies, CM has greater potential for practical applications, but the premise is that the chemical stability of pre-lithium-intercalated anode materials should be addressed.
4.2 Lithium source added to the positive electrode
This approach is an effective strategy for mitigating initial ALL in practical applications. But the lithium source should satisfy several requirements at the same time, namely chemical stability, high capacity, suitable potential range, and good electrical conductivity. Additionally, the microstructure of the electrodes needs to be carefully designed and tested when using this method.
4.3 Sacrificial electrode method
This method has been widely used in the fabrication of LICs, but not in the field of LIBs. The capacity of battery electrodes is several times higher than that of LICs, and the electrodes are well matched, and the pre-intercalation of lithium needs to be handled more uniformly and accurately to ensure safe cycling. Therefore, it is necessary to carefully control the pre-lithiation degree and rate, which involves pore density and pore size design on current collectors and electrodes, optimization of the resting time required for ion diffusion to equilibrium, and other detailed parameter optimizations.
4.4 Additional Lithium Sources
The small particle size and relatively low purity (<98%) of SLMP bring some difficulties to coating and assembly and negatively affect the electrochemical performance. For comparison with the pre-lithium intercalation strategy of LICs, four different types of Li source structures are introduced, namely SLMP, thick Li strip, thin Li foil, and thin Li foil with pinholes. Pinholes in the thin lithium foil help electrolyte flow to the electrodes and allow gas to escape from the electrodes. Compared with using SLMP, the use of Li foil for pre-intercalation results in a more efficient and safer battery, and since Li+ is derived from the Li surface, the high surface area of the Li source (e.g. SLMP) results in higher pre-intercalation of Li Rate. But the thickness of Li foil for LICs is 15 mm or thicker, which is still not enough for pre-intercalation due to huge production difficulties in industry. The commercialization of thinner lithium foils (< 10 um) is one of the prerequisites for the practical application of this method.

