Lithium-ion battery cathode materials currently used in commercial applications mainly include lithium manganate (LiMn2O4), lithium iron phosphate (LiFePO4), lithium cobalt oxide (LiCoO2), nickel cobalt lithium manganate (NCM) and nickel cobalt lithium aluminate (NCA) . The actual specific capacity of the above materials is between 110 and 200 mA h/g, but the specific capacity of the commercialized negative electrode material is very high, which is about 3 to 20 times the specific capacity of the positive electrode material. Cathode materials are becoming a bottleneck for further improving the performance of lithium-ion batteries.
In 2004, the Argonne Laboratory in the United States synthesized xLi2MnO3·(1-x)LiMO2 (M=Ni, Co, Mn) lithium-rich manganese-based cathode materials for the first time. This material has high specific capacity (≈ 250 mAh/g), high Due to the advantages of operating voltage (≈ 3.6 V) and low cost, it is expected to become the cathode material for next-generation commercial high-energy-specific lithium batteries.
Defects of Li-rich Manganese-Based Cathode Materials
(1) Large reversible capacity loss and low Coulombic efficiency for the first charge and discharge
The study believes that when the first charging voltage is higher than 4.5V, the inactive Li2MnO3 component in the lithium-rich manganese-based cathode material is activated and participates in the reaction, and the O2- in the lattice changes from Li2MnO3 in the form of “Li2O”. The component overflowed, and the remaining part became the new component MnO2.
The activation of the Li2MnO3 phase during the first charge will generate lithium-containing compounds, and this process can only be carried out in one direction, resulting in a very large loss of capacity during the first charge, which in turn leads to a low first-time Coulombic efficiency.
In addition, the positive electrode material will also react with Li+ in the electrolyte during charging, and the reaction will form an SEI film, especially when the voltage is higher than 4.5V, the irreversibility of the reaction will be increased, resulting in an increase in Li+ loss, which is also the first cause of the material. One of the factors that reduces the coulombic efficiency of one charge and discharge.
(2) Serious attenuation of capacity and voltage
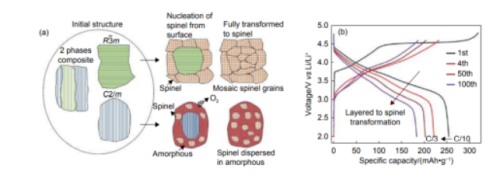
The biggest problem of Li-rich manganese-based cathode materials is voltage and capacity fading. The voltage decay is due to the change of its bulk phase structure, the material loses the lattice oxygen on the surface during charging, the transition metal ions in the material migrate due to the reduction of the coordination number of oxygen, and the crystal changes from a layered structure to a spinel structure. , and eventually into a disordered rock-salt structure. The loss of lattice oxygen causes the internal Ni and Mn transition metal elements to move to the surface, so the surface layer of the material presents a spinel phase reconstruction layer deficient in Li, multi-Mn and Ni. In addition, the escaped oxygen will react with the electrolyte. As the number of reactions increases, the reaction between the lattice oxygen and the electrolyte becomes more and more intense, resulting in the spinel phase extending from the surface of the material to the middle one after another. It exacerbates the problem of material voltage and capacity fading.
(3) Poor rate performance
After the first cycle of Li-rich manganese-based materials, some of them are added to the redox reaction in the LiMnO2 structure, so the stability is not good. At the same time, the ion diffusivity of the material is low, resulting in relatively poor rate performance. In addition, the coexistence of the electrode material and the electrolyte makes the lattice oxygen on the surface of the material more active. On the one hand, the release of oxygen will reduce the specific capacity of the material, and on the other hand, oxygen will also act on the electrolyte to reduce the reversible Li+ in it. The generated irreversible compounds accumulate on the surface of the electrode, hinder the migration of Li+ from the surface of the electrode material to the interior, and directly lead to the poor rate performance of the lithium-rich manganese-based cathode material.
(a) Schematic diagram of structural evolution of Li-rich manganese-based cathode material; (b) charge-discharge curve
Modification of Li-rich Manganese-Based Cathode Materials
In order to solve the defect problem of Li-rich manganese-based materials, the process of improving the stability of materials by means of ion doping and surface coating has been relatively mature. In addition, in recent years, some novel modification strategies such as surface modification, morphology, and component design have achieved remarkable results.
(1) Ion doping
The principle of ion doping modification: stabilize the crystal structure of the cathode material, slow down the migration and mixing of transition metal ions during the cycle, improve the electrochemical cycle stability, and form M-O with stronger bond energy inside the cathode material lattice bond, while promoting Li+ migration, improving the conductivity of lithium ions inside the cathode material, and finally the first Coulombic efficiency of the lithium-rich manganese-based cathode material is improved. At present, the main forms of ion doping include cation doping, anion doping, polyanion doping and co-doping.
(2) Surface coating
The surface coating can effectively protect the electrode material, inhibit the interface side reaction between the positive electrode particles and the electrolyte, and at the same time, it can block the release of oxygen to a certain extent, improve the reversible capacity, and improve the cycle performance. According to the mechanism of action, the coating can be divided into inert coating (such as MgO, SnO2, CeO2, AlF3, CoF2, MgF2, CePO4), electronic conductive coating (such as polyethylene dioxythiophene), ion conductive coating (such as Li2ZrO3, Li4Ti5O12, LiCoPO4) and active coating layers (such as MnO2, Nb2O5, Co3O4).
(3) Surface modification
Heterostructures: Introducing spinel phases into layered Li-rich manganese-based materials is an effective way to suppress their lattice oxygen release at high voltages. The spinel phase has high compatibility with the layered phase, and the spinel phase can provide a three-dimensional channel for the migration of Li+, shorten the migration path of Li+, and comprehensively improve the kinetic properties and thermodynamic stability of the material.
Constructing oxygen vacancies: Oxygen vacancies play a role in stabilizing the crystal structure, regulating ion mobility, and improving electronic conductivity in Li-rich manganese-based materials. The introduction of appropriate oxygen vacancies helps to suppress the irreversible oxygen extraction from the material surface and slow down the voltage decay. According to first-principles calculations, oxygen is more likely to undergo the O2-→O- reaction in the presence of oxygen vacancies, and the improvement of reversible oxygen activity is the key to inhibiting the phase transition of materials.
(4) Morphology and composition design
Morphology design: The preparation of layered Li-rich manganese-based materials into nanomaterials can help shorten Li+ migration paths, improve element distribution and overall chemical environment. However, nanomaterials have a large specific surface area and are easily corroded by electrolytes, resulting in a phase transition of the material and ultimately voltage decay. Therefore, alleviating the corrosion of the electrolyte to the material is the key to exerting the advantages of nanomaterials. And because the Li+ transport rate directly determines its rate capability, if the exposed crystal planes of the crystal can be controlled during the synthesis process, the rate capability can be improved to a certain extent.
Conclusion and Outlook
With the development of society, electric vehicles, 3C electronic products, energy storage devices, etc. have put forward higher requirements on the energy density of lithium-ion batteries. The lithium-rich manganese-based cathode material has the advantages of high specific capacity and low cost, and the discharge specific capacity is over 250mA·h/g, which will be the technical key for the future lithium battery to reach 400W·h/kg or even 500W·h/kg. Very high application prospect.
However, some defects of Li-rich manganese-based materials still need to be solved urgently. At present, there are many methods to deal with them, including ion doping, surface coating, surface modification, morphology and component design. However, the above methods are only aimed at improving the performance of materials in a single aspect, and there is no sure-fire solution. It is expected that in the future, we can combine various modification methods to find the best modification scheme to comprehensively improve the performance of Li-rich manganese base.