The enhanced structural stability of Gr, thus increasing the energy density and reducing the volume expansion. Therefore, pairing a high-Ni cathode with a Si/Gr composite anode is expected to provide high-energy-density Li-ion batteries for automotive applications. However, the dissolution of transition metals (TMs) from NMC cathodes and their subsequent deposition on the anode surface has been considered a prominent capacity fading mechanism in LIBs and Li metal batteries.
It has been reported that TM dissolution is mainly caused by the attack of acidic species, such as hydrofluoric acid (HF), which results from the hydrolysis or thermal decomposition of lithium hexafluorophosphate (LiPF6) salts or the electrochemical oxidation of LiPF6-based high-voltage electrolytes. In addition, the dissolution of TM ions was also found in NMC811 cathode with lithium bis(fluorosulfonyl)imide (LiFSI)-1,2-dimethoxyethane (DME) electrolyte. This may be attributed to other mechanisms for triggering TM dissolution proposed by Evertz et al: i) impurities within the NMC host lattice introduced during synthesis, ii) increasing surface exposure due to particle fracture, and iii) Phase transitions during deep delithiation. Recently, Sahore et al. proposed an alternative TM dissolution mechanism by performing chemical aging experiments on NMC532 cathodes in the presence of different ligands, anions, and solvents. They demonstrated that at low voltage, the main dissolution mechanism of TM is HF attack; however, at high voltage, the main mechanism is due to the oxidative decomposition of the electrolyte on the surface of the cathode, and the formation of solubility through the cation exchange reaction between the cathode and the liquid electrolyte. TM complexes, leading to partial reduction of TM.
The dissolved TM ions will further migrate and deposit on the anode surface, which has been widely observed in batteries with various NMC-based cathodes. Using time- and space-resolved X-ray absorption spectroscopy, Wandt et al. demonstrated that the oxidation states of deposited Ni, Mn, and Co ions are +2 on both lithiated and delithiated Gr anodes. These deposited TM ions undergo a reduction reaction through the TM-Li ion exchange mechanism on the surface of the negative electrode, and two Li+ ions are extracted from the negative electrode. Furthermore, it has been reported that the accumulated TM ions on the anode surface catalyze solvent decomposition, leading to the formation of an organic-rich anode-electrolyte interface (AEI) layer on the Gr surface. Jung et al. compared the effects of Ni, Mn, and Co deposition on the electrochemical performance of Gr||NMC622 cells. They report that the deposited Mn exhibits higher activity towards AEI decomposition compared to Ni and Co, resulting in more cyclable Li loss and worse cycling performance. Moreover, Solchenbach et al. reported that the reduced manganese deposited on the anode surface could decompose the organic components in AEI, impair the passivation performance of AEI and initiate a continuous electrolyte reduction reaction. All of the above adverse reactions consume active Li, leading to a significant increase in anode impedance, severely reducing the intercalation reversibility and diffusivity of Li+, and ultimately leading to rapid capacity decay. It was reported that the capacity retention of Gr||NMC622 cells after 298 cycles at 4.2V was reduced by 4.8%, 13.7% and 4.8%, respectively, compared with the control electrolyte when 30 mM of Ni2+, Mn2+ and Co2+ were additionally introduced into the electrolyte, respectively. 7.3%.
1. Introduction to the results
Silicon anodes are considered as one of the most likely replacements for graphite (Gr) anodes due to their ultra-high capacity, abundance, and low cost. Coupling the Si-based anode with the high-Ni layered oxide cathode LiNixMnyCo1−x−yO2 (NMC, x ≥ 0.8) can improve the driving range of electric vehicles. Transition metal (TM) ion dissolution and deposition is one of the failure causes of Gr-based Li-ion batteries. However, few mechanistic insights related to the deposition of TM ions on Si-based anodes have been reported. Here, the work published in Adv.EnergyMater. by ArumugamManthiram’s team from the University of Texas at Austin investigated the effect of in-situ deposited TM ions on the SiOx/Gr composite anode and the effects of Ni, Mn and Co on the structure and electrochemistry Stability effects and decay mechanisms. The detrimental effect of TM ion dissolution on Si is greater than that on Gr, and different TM ions have different effects on the formation of the anode-electrolyte interface. Specifically, Ni deposition induces more violent salt decomposition; Co deposition has negligible effect on salt decomposition but significantly accelerates solvent decomposition; Mn deposition exacerbates both salt and solvent decomposition, resulting in worse full-cell performance. The degree of degradation decreases in the order of Mn2+>Ni2+>Co2+, and the proposed system comparison can guide the further development of high-energy systems.
2. Research highlights
Although the crosstalk between TM dissolution and Gr-based anodes has been extensively studied, the mechanism related to TM dissolution from high-Ni cathodes and deposition on Si-based anodes is rarely reported. Therefore, it is crucial to elucidate the dissolution and deposition of TM, revealing its influence mechanism on Si-based anodes and the corresponding battery performance. In this work, the effects of TM dissolution on the stability of SiOx/Gr composite anodes and cells paired with ultra-high nickel cathodes LiNi0.90Mn0.05Co0.05O2 (NMC955) via in-situ deposition of TM ions on the surface of SiOx/Gr anodes An evaluation was carried out. Then, the effects of dissolved Ni, Mn and Co on the structure and electrochemical stability of SiOx/Gr||NMC955 cells were investigated by introducing additional TM ions into the electrolyte and compared with Gr||NMC955 cells . The results show that the dissolved TM ions have a clear detrimental effect on the battery performance, and the degradation of SiOx/Gr is stronger than that of Gr. X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) characterizations were used to reveal the underlying mechanisms of the varying degrees of degradation of the deposits with different TM ions. An in-depth understanding of how TM dissolution affects Si-based anodes will shed light on strategies to control this crossover effect and will facilitate the practical application of high-energy-density Li-ion batteries with high-Ni cathodes and Si-based anodes.
3. Picture and text guide
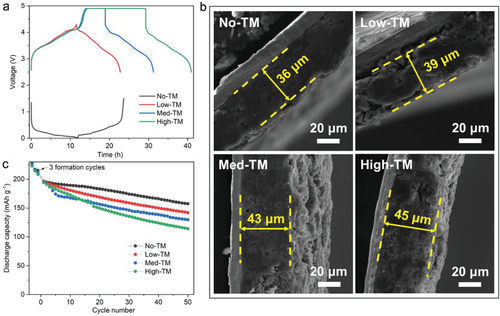
It is reported that the amount of TM ions dissolved from the layered oxide cathode and subsequently deposited on the Gr anode is proportional to the upper cut-off voltage, and the dissolution rate will accelerate when 4.6 V is exceeded. It has been reported that the effect of TM ions on SiOx/Gr anodes and the corresponding full-cell cycling stability, SiOx/Gr anodes with different numbers of TM ions deposited on the surface were prepared by controlling the upper cut-off voltage and high voltage holding time. As shown in Fig. 1a, SiOx/Gr anodes without TM ions on the surface (designated as No- TM). The SiOx/Gr anode was obtained by charging the SiOx/Gr||NMC955 cells to 4.3 V, respectively, to obtain deposited TM ions with low, medium and high content (denoted as Low-TM, Med-TM and High-TM, respectively) without any retention time, 4.9V hold time for 5 hours, 4.9V hold time for 15 hours, then each of the three cells was discharged to 2.5V with two cycles in the voltage range of 2.5-4.3V. Using inductively coupled plasma analysis, the contents of Ni, Mn and Co ions deposited on the surface of the SiOx/Gr anode were quantified as 109, 68 and 51 ppm for Low-TM; 529, 314 and 219 ppm for Med-TM; -TM were 1585, 893 and 747ppm, respectively. The metal content is calculated based on the mass of the SiOx/Gr material within the SiOx/Gr electrode.
The effect of TM ions on electrode swelling after the deposition process was evaluated by scanning electron microscopy (SEM). Figure 1b shows the cross-sectional SEM images of the SiOx/Gr composite electrodes deposited with different TM ion contents. The average thickness of SiOx/Gr electrodes without TM ion deposition is about 36 µm, while the average electrode thicknesses with TM ion deposition are ≈39, ≈43, and ≈45 µm for Low-TM, Med-TM, and High-TM electrodes, respectively. The results show that the irreversible electrode expansion is exacerbated with the increase of the TM ion content deposited on the SiOx/Gr anode surface. For Gr-based LIBs, dissolved TM ions have been shown to catalyze the decomposition of electrolytes, especially solvent reduction in conventional carbonate-based electrolytes, which leads to the formation of unstable electrolyte-electrode interfacial layers on the Gr surface. Likewise, the increased electrode swelling in SiOx/Gr after deposition of TM ions may be due to the accelerated decomposition of the electrolyte catalyzed by TM ions on the electrode surface, leading to the formation of the dominant organic species AEI. Such AEIs are typically thick, non-uniform, and unprotected to suppress continuous electrolyte decomposition during cycling. It can also be speculated that due to the slow Li diffusion through this degraded AEI, more active Li can be trapped in the SiOx/Gr anode, resulting in a larger volume change of the Si-based anode after cycling. The increased electrode swelling will be detrimental to the stability of the SiOx/Gr electrode and correspondingly affect the battery performance during cycling. This is further supported by the poor cycling stability of the SiOx/Gr||NMC955 cell with SiOx/Gr with higher deposited TM ion content, as shown in Figure 1c.
To investigate the effects of Ni, Mn, and Co on the electrochemical performance of Si/Gr composite anode and high-Ni cathode cells, specific amounts of TM ions were introduced into the electrolyte from outside. According to previous reports, 1 M LiPF6/EC-EMC (3:7 wt.) containing 10 wt.% FEC (referred to as LFEC) was used as the control electrolyte. 20 mM Ni(TFSI)2, Mn(TFSI)2 and Co(TFSI)2 were mixed with LFEC and labeled LFN, LFM and LFC, respectively. Furthermore, to investigate the effect of introducing additional TFSI- by TM(TFSI)2, an electrolyte called LFL was also prepared in LFEC using 40 mM LiTFSI. Since the studied SiOx/Gr is a composite material, the cycling performance of the Gr||NMC955 battery was also evaluated using five electrolytes. Figure 2a illustrates the initial Coulombic efficiencies (CE) of SiOx/Gr||NMC955 and Gr||NMC955 cells in different electrolytes. The CEs of SiOx/Gr||NMC955 and Gr||NMC955 cells with LFEC and LFL are found to be nearly identical, indicating that the additionally introduced TFSI- has negligible effects on the electrode-electrolyte interface formation and the diffusion of Li+ during the initial formation cycle. This can be further confirmed by the similar cycling stability of the SiOx/Gr||NMC955 with LFL electrolyte (Fig. 2b) and the Gr||NMC955 cell with the cell with LFEC electrolyte. The initial CE of the SiOx/Gr||NMC955 and Gr||NMC955 cells decreased significantly when various TM ions were incorporated into the electrolyte, and the cells with LFM and LFC exhibited the lowest and highest among the three TM ion-containing electrolytes, respectively CE. These observations suggest that different TM ions have different detrimental effects on the formation of the electrode-electrolyte interface as well as the electrochemical behavior of the battery. This is further supported by the observation of the dQ/dV curves, it can be seen that the redox peak potentials of LFN, LFM and LFC cells show a larger difference than LFEC and LFL cells, implying that TM ions are transported after the introduction of TM ions into the cells. Kinetics are slow. Furthermore, it should be noted that although the initial CE of SiOx/Gr||NMC955 cells with LFEC and LFL was slightly higher than that of Gr||NMC955 cells with LFEC and LFL, this trend did not extend to the use of LFN, LFM and SiOx/Gr||NMC955 and Gr||NMC955 cells with LFC electrolytes. This difference suggests that the adverse effect of TM ions should be more pronounced on SiOx/Gr than on the Gr anode.
In this study, the detrimental effects of TM ion dissolution on the cycling performance of silicon-based anodes paired with high-Ni cathodes and the underlying degradation mechanisms are first comprehensively investigated. By comparing the Gr||NMC955 cells, it is proved that the influence of TM ions deposited on Si is much larger than that of Gr. The SiOx/Gr anode is more easily degraded by TM ion deposits than the NMC955 cathode, thus more likely to lead to the low CE and rapid capacity fading of the SiOx/Gr||NMC955 battery. The degree of adverse effects of TM ions on Si-based electrodes increases in the order of Co<Ni<Mn. This trend is mainly due to the different roles of the deposited TM ions in the AEI formation on the SiOx/Gr surface. TOF-SIMS analysis showed that Co ion deposits had limited effect on the decomposition reaction of LiPF6 salts, but had a significant effect on solvolysis, resulting in more organic AEI layers. Conversely, the deposition of Ni ions is more likely to accelerate the decomposition of LiPF6 salts. On the other hand, Mn ion deposits not only accelerate solvent decomposition but also induce violent decomposition of LiPF6, resulting in the thickest AEI layer with high resistance and poor protection. These results establish fundamental guidelines for controlling the cross-effect phenomenon and help facilitate the design of high-energy-density Li-ion batteries with high-Ni cathodes and Si-based anodes for electric vehicles.
References
Zhang,X. H., Cui, Z. H. & Manthiram, A. Insights into the CrossoverEffects in Cells with High-Nickel Layered Oxide Cathodes andSilicon/Graphite Composite Anodes. Adv. Energy Mater. 2022.